The Triple Helix of Collagens – an Ancient Protein Structure That Enabled Animal Multicellularity and Tissue Evolution Aaron L
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Flexible Fluidic Actuators for Soft Robotic Applications
University of Wollongong Research Online University of Wollongong Thesis Collection 2017+ University of Wollongong Thesis Collections 2019 Flexible Fluidic Actuators for Soft Robotic Applications Weiping Hu Follow this and additional works at: https://ro.uow.edu.au/theses1 University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Research Online is the open access institutional repository for the University of -

Ancient Metaproteomics: a Novel Approach for Understanding Disease And
Ancient metaproteomics: a novel approach for understanding disease and diet in the archaeological record Jessica Hendy PhD University of York Archaeology August, 2015 ii Abstract Proteomics is increasingly being applied to archaeological samples following technological developments in mass spectrometry. This thesis explores how these developments may contribute to the characterisation of disease and diet in the archaeological record. This thesis has a three-fold aim; a) to evaluate the potential of shotgun proteomics as a method for characterising ancient disease, b) to develop the metaproteomic analysis of dental calculus as a tool for understanding both ancient oral health and patterns of individual food consumption and c) to apply these methodological developments to understanding individual lifeways of people enslaved during the 19th century transatlantic slave trade. This thesis demonstrates that ancient metaproteomics can be a powerful tool for identifying microorganisms in the archaeological record, characterising the functional profile of ancient proteomes and accessing individual patterns of food consumption with high taxonomic specificity. In particular, analysis of dental calculus may be an extremely valuable tool for understanding the aetiology of past oral diseases. Results of this study highlight the value of revisiting previous studies with more recent methodological approaches and demonstrate that biomolecular preservation can have a significant impact on the effectiveness of ancient proteins as an archaeological tool for this characterisation. Using the approaches developed in this study we have the opportunity to increase the visibility of past diseases and their aetiology, as well as develop a richer understanding of individual lifeways through the production of molecular life histories. iii iv List of Contents Abstract ............................................................................................................................... -

Environmentally Controlled Curvature of Single Collagen Proteins
Environmentally controlled curvature of single collagen proteins Naghmeh Rezaei, Aaron Lyons and Nancy R. Forde* Department of Physics Simon Fraser University 8888 University Drive Burnaby, BC V5A 1S6 CANADA *Corresponding author. 778-782-3161. [email protected]. ORCID: 0000-0002-5479-7073 - 1 - ABSTRACT The predominant structural protein in vertebrates is collagen, which plays a key role in extracellular matrix and connective tissue mechanics. Despite its prevalence and physical importance in biology, the mechanical properties of molecular collagen are far from established. The flexibility of its triple helix is unresolved, with descriptions from different experimental techniques ranging from flexible to semirigid. Furthermore, it is unknown how collagen type (homo- vs. heterotrimeric) and source (tissue-derived vs. recombinant) influence flexibility. Using SmarTrace, a chain tracing algorithm we devised, we performed statistical analysis of collagen conformations collected with atomic force microscopy (AFM) to Our results show that types I, II and III collagens the key fibrillar varieties exhibit molecular flexibilities that are very similar. However, collagen conformations are strongly modulated by salt, transitioning from compact to extended as KCl concentration increases, in both neutral and acidic pH. While analysis with a standard worm-like chain model suggests that the persistence length of collagen can attain almost any value within the literature range, closer inspection reveals that this modulation to changes in flexibility, but rather arises from the induction of curvature (either intrinsic or induced by interactions with the mica surface). By modifying standard polymer theory to include innate curvature, we show that collagen behaves as an equilibrated curved worm-like chain (cWLC) in two dimensions. -

The Frog in Taffeta Pants
Evolutionary Anthropology 13:5–10 (2004) CROTCHETS & QUIDDITIES The Frog in Taffeta Pants KENNETH WEISS What is the magic that makes dead flesh fly? himself gave up on the preformation view). These various intuitions arise natu- Where does a new life come from? manded explanation. There was no rally, if sometimes fancifully. The nat- Before there were microscopes, and compelling reason to think that what uralist Henry Bates observed that the before the cell theory, this was not a one needed to find was too small to natives in the village of Aveyros, up trivial question. Centuries of answers see. Aristotle hypothesized epigenesis, the Tapajos tributary to the Amazon, were pure guesswork by today’s stan- a kind of spontaneous generation of believed the fire ants, that plagued dards, but they had deep implications life from the required materials (pro- them horribly, sprang up from the for the understanding of life. The vided in the egg), that systematic ob- blood of slaughtered victims of the re- 5 phrase spontaneous generation has servation suggested coalesced into a bellion of 1835–1836 in Brazil. In gone out of our vocabulary except as chick. Such notions persisted for cen- fact, Greek mythology is full of beings an historical relic, reflecting a total turies into what we will see was the spontaneously arising—snakes from success of two centuries of biological critical 17th century, when the follow- Medusa’s blood, Aphrodite from sea- research.1 The realization that a new ing alchemist’s recipe was offered for foam, and others. Even when the organism is always generated from the production of mice:3,4 mix sweaty truth is known, we can be similarly one or more cells shed by parents ex- underwear and wheat husks; store in impressed with the phenomena of plained how something could arise open-mouthed jar for 21 days; the generation. -

Peptoid Residues Make Diverse, Hyperstable Collagen Triple Helices
Peptoid Residues Make Diverse, Hyperstable Collagen Triple Helices Julian L. Kessler1, Grace Kang1, Zhao Qin2, Helen Kang1, Frank G. Whitby3, Thomas E. Cheatham III4, Christopher P. Hill3, Yang Li1,*, and S. Michael Yu1,5 1Department of Biomedical Engineering, University of Utah, Salt Lake City, Utah 84112, USA 2Department of Civil & Environmental Engineering, Collagen of Engineering & Computer Science, Syracuse University, Syracuse, New York 13244, USA 3Department of Biochemistry, University of Utah School of Medicine, Salt Lake City, UT 84112, USA 4Department of Medicinal Chemistry, College of Pharmacy, L. S. Skaggs Pharmacy Research Institute, University of Utah, Salt Lake City, Utah 84112, USA 5Department of Pharmaceutics and Pharmaceutical Chemistry, University of Utah, Salt Lake City, Utah 84112, USA *Corresponding Author: Yang Li ([email protected]) Abstract The triple-helical structure of collagen, responsible for collagen’s remarkable biological and mechanical properties, has inspired both basic and applied research in synthetic peptide mimetics for decades. Since non-proline amino acids weaken the triple helix, the cyclic structure of proline has been considered necessary, and functional collagen mimetic peptides (CMPs) with diverse sidechains have been difficult to produce. Here we show that N-substituted glycines (N-glys), also known as peptoid residues, exhibit a general triple-helical propensity similar to or greater than proline, allowing synthesis of thermally stable triple-helical CMPs with unprecedented sidechain diversity. We found that the N-glys stabilize the triple helix by sterically promoting the preorganization of individual CMP chains into the polyproline-II helix conformation. Our findings were supported by the crystal structures of two atomic-resolution N-gly-containing CMPs, as well as experimental and computational studies spanning more than 30 N-gly-containing peptides. -
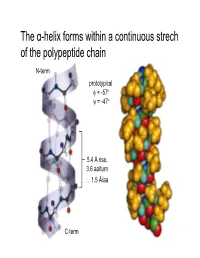
The Α-Helix Forms Within a Continuous Strech of the Polypeptide Chain
The α-helix forms within a continuous strech of the polypeptide chain N-term prototypical φ = -57 ° ψ = -47 ° 5.4 Å rise, 3.6 aa/turn ∴ 1.5 Å/aa C-term α-Helices have a dipole moment, due to unbonded and aligned N-H and C=O groups β-Sheets contain extended (β-strand) segments from separate regions of a protein prototypical φ = -139 °, ψ = +135 ° prototypical φ = -119 °, ψ = +113 ° (6.5Å repeat length in parallel sheet) Antiparallel β-sheets may be formed by closer regions of sequence than parallel Beta turn Figure 6-13 The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation • Interactions between adjacent R-groups – Ionic attraction or repulsion – Steric hindrance of adjacent bulky groups Helix wheel The stability of helices and sheets depends on their sequence of amino acids • Intrinsic propensity of an amino acid to adopt a helical or extended (strand) conformation • Interactions between adjacent R-groups – Ionic attraction or repulsion – Steric hindrance of adjacent bulky groups • Occurrence of proline and glycine • Interactions between ends of helix and aa R-groups His Glu N-term C-term -

Learning in the Land Snail (Helix Aspers a Mliller)*
Bulletin of the Psychonomic Society 1974, Vol. 4 (SA), 476-478 Learning in the land snail (Helix aspers a Mliller)* R. K. SIEGEL and M. E. JARVIK Departments of Pharmacology and Psychiatry, University of California, Los Angeles Los Angeles, California 90024 A group of two experiments are discussed which demonstrate avoidance learning in land snails. Experiment I utilized the snail's negative geotaxis and its chemoreceptive characteristics and required the snail to climb a vertical pole which contained a quinine-saturated loop of thread at the top. Experiment II substituted electric shock loops for the quinine. Snails in both experimental groups manifested progressively increasing climbing latencies and avoidance responses throughout five successive training sessions and a one week retention test. Control animals which received noncontingent quinine or shock did not show evidence of learning. These results provide evidence of rapid avoidance learning in gastropod mollusks. Very few investigators have examined the problem of employed a pole-climbing response with quinine as an learning in snails (see reviews by Stephens & McGaugh, aversive stimulus at the top of the pole. These authors 1972; Willows, 1973). Early workers have suggested that demonstrated both short- and long-term memory of the these gastropods show evidence of classical conditioning aversive experience as well as habituation of the climbing (Thompson, 1917), maze learning (Garth & Mitchell, response itself with a neutral water stimulus. This 1926; Fischel, 1931), and habituation (Humphrey, learning appeared to be modifiable by length of 1930; Grindley, 1937). The phenomenon of classical laboratory housing, humidity, nutrition, and conditioning in snails has been recently reexamined by reproductive conditions. -

Decelerated Genome Evolution in Modern Vertebrates Revealed by Analysis of Multiple Lancelet Genomes
ARTICLE Received 20 May 2014 | Accepted 18 Nov 2014 | Published 19 Dec 2014 DOI: 10.1038/ncomms6896 OPEN Decelerated genome evolution in modern vertebrates revealed by analysis of multiple lancelet genomes Shengfeng Huang1, Zelin Chen1, Xinyu Yan1, Ting Yu1, Guangrui Huang1, Qingyu Yan1, Pierre Antoine Pontarotti2, Hongchen Zhao1, Jie Li1, Ping Yang1, Ruihua Wang1, Rui Li1, Xin Tao1, Ting Deng1, Yiquan Wang3,4, Guang Li3,4, Qiujin Zhang5, Sisi Zhou1, Leiming You1, Shaochun Yuan1, Yonggui Fu1, Fenfang Wu1, Meiling Dong1, Shangwu Chen1 & Anlong Xu1,6 Vertebrates diverged from other chordates B500 Myr ago and experienced successful innovations and adaptations, but the genomic basis underlying vertebrate origins are not fully understood. Here we suggest, through comparison with multiple lancelet (amphioxus) genomes, that ancient vertebrates experienced high rates of protein evolution, genome rearrangement and domain shuffling and that these rates greatly slowed down after the divergence of jawed and jawless vertebrates. Compared with lancelets, modern vertebrates retain, at least relatively, less protein diversity, fewer nucleotide polymorphisms, domain combinations and conserved non-coding elements (CNE). Modern vertebrates also lost substantial transposable element (TE) diversity, whereas lancelets preserve high TE diversity that includes even the long-sought RAG transposon. Lancelets also exhibit rapid gene turnover, pervasive transcription, fastest exon shuffling in metazoans and substantial TE methylation not observed in other invertebrates. These new lancelet genome sequences provide new insights into the chordate ancestral state and the vertebrate evolution. 1 State Key Laboratory of Biocontrol, Guangdong Key Laboratory of Pharmaceutical Functional Genes, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China. 2 Evolution Biologique et Mode´lisation UMR 7353 Aix Marseille Universite´/CNRS, 3 Place Victor Hugo, 13331 Marseille, France. -

Photoperiod and Temperature Interaction in the Helix Pomatia
Photoperiod and temperature interaction in the determination of reproduction of the edible snail, Helix pomatia Annette Gomot Laboratoire de Zoologie et Embryologie, UA CNRS 687, Faculté des Sciences et Techniques et Centre Universitaire d'Héliciculture, Université de Franche-Comté, 25030 Besançon Cedex, France Summary. Snails were kept in self-cleaning housing chambers in an artificially con- trolled environment. Mating was frequent under long days (18 h light) and rare under short days (8 h light) regardless of whether the snails were kept at 15\s=deg\Cor 20\s=deg\C.An interaction between photoperiod and temperature was observed for egg laying. The number of eggs laid (45\p=n-\50/snail)and the frequency of egg laying (90\p=n-\130%)were greater in long than in short days (16\p=n-\35/snailand 27\p=n-\77%)but a temperature of 20\s=deg\C redressed, to some extent, the inhibitory effect of short days. At both temperatures only long photoperiods brought about cyclic reproduction over a period of 16 weeks, con- firming the synchronizing role of photoperiod on the neuroendocrine control of egg laying in this species of snail. Keywords: edible snail; mating; egg laying; photoperiod; temperature Introduction The effects of photoperiod and temperature, which influence most reproductive cycles of animals of temperate regions, have given rise to a certain number of observations for pulmonate gastropods. In basommatophorans, the influence of photoperiod and temperature on reproduction has been shown in four species of lymnaeids and planorbids (Imhof, 1973), in Melampus (Price, 1979), in Lymnaea stagnalis (Bohlken & Joosse, 1982; Dogterom et ai, 1984; Joosse, 1984) and in Bulinus truncatus (Bayomy & Joosse, 1987). -

Stream Inventory Handbook: Level 1 and Level II
Stream Inventory Handbook: Level 1 and Level II CONTENTS CHAPTER 1 Introduction/Overview ............................................................................................................ 3 Background ......................................................................................................................................... 3 Inventory Attributes ............................................................................................................................ 4 Establishing Forest Priorities .............................................................................................................. 5 Stream Inventory Program Management ............................................................................................ 5 Program Administration And Quality Control .................................................................................... 6 A Standard Protocol ............................................................................................................................ 7 Presentation of Information ................................................................................................................. 8 Handbook Content .............................................................................................................................. 9 CHAPTER 2 Office Procedures Level I Inventory - Identification Level .................................................. 12 Objectives ......................................................................................................................................... -

Proteomic Strategies for Cultural Heritage: from Bones to Paintings☆
Microchemical Journal 126 (2016) 341–348 Contents lists available at ScienceDirect Microchemical Journal journal homepage: www.elsevier.com/locate/microc Proteomic strategies for cultural heritage: From bones to paintings☆ Roberto Vinciguerra a, Addolorata De Chiaro a, Piero Pucci a, Gennaro Marino b, Leila Birolo a,⁎ a Dipartimento di Scienze Chimiche, Università di Napoli Federico II, Complesso Universitario Monte S.Angelo, Via Cinthia, Napoli, Italy b Università degli Studi ‘Suor Orsola Benincasa’, 80132 Napoli, Italy article info abstract Article history: In recent years, proteomics procedures have become increasingly popular for the characterization of proteina- Received 30 July 2015 ceous materials in ancient samples of several cultural heritage objects. The knowledge of the materials used in Received in revised form 18 December 2015 a work of art is crucial, not only to give an insight in the historical context of objects and artists, but also to analyse Accepted 18 December 2015 degradation processes taking place in aged objects and to develop appropriate conservation and/or restoration Available online 29 December 2015 treatments. However, protocols routinely applied for typical modern samples still need to be fully adapted to Keywords: take into account the low amount of proteinaceous material, the heterogeneity and the unusual physical state Proteomics of the samples, as well as the high levels of damage found in ancient samples. This paper deals with some exam- Ancient proteins ples of the adaptation of classical proteomic strategies in the analysis of ancient samples to meet the different LC-MS/MS aims in the cultural heritage field. © 2015 Elsevier B.V. All rights reserved. -

Analysis of 5000 Year-Old Human Teeth Using Optimized Large-Scale And
Analysis of 5000 year-old human teeth using optimized large-scale and targeted proteomics approaches for detection of sex-specific peptides Carine Froment, Mathilde Hourset, Nancy Sáenz-Oyhéréguy, Emmanuelle Mouton-Barbosa, Claire Willmann, Clément Zanolli, Rémi Esclassan, Richard Donat, Catherine Thèves, Odile Burlet-Schiltz, et al. To cite this version: Carine Froment, Mathilde Hourset, Nancy Sáenz-Oyhéréguy, Emmanuelle Mouton-Barbosa, Claire Willmann, et al.. Analysis of 5000 year-old human teeth using optimized large-scale and targeted proteomics approaches for detection of sex-specific peptides. Journal of Proteomics, Elsevier, 2020, 211, pp.103548. 10.1016/j.jprot.2019.103548. hal-02322441 HAL Id: hal-02322441 https://hal.archives-ouvertes.fr/hal-02322441 Submitted on 18 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Journal Pre-proof Analysis of 5000year-old human teeth using optimized large- scale and targeted proteomics approaches for detection of sex- specific peptides Carine Froment, Mathilde Hourset, Nancy Sáenz-Oyhéréguy, Emmanuelle Mouton-Barbosa, Claire Willmann, Clément Zanolli, Rémi Esclassan, Richard Donat, Catherine Thèves, Odile Burlet- Schiltz, Catherine Mollereau PII: S1874-3919(19)30320-3 DOI: https://doi.org/10.1016/j.jprot.2019.103548 Reference: JPROT 103548 To appear in: Journal of Proteomics Received date: 24 January 2019 Revised date: 30 August 2019 Accepted date: 7 October 2019 Please cite this article as: C.