(Vles) in the Yeast Debaryomyces Hansenii
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Killer Toxin of Saccharomyces Cerevisiae Y500-4L Active Against Fleischmann and Itaiquara Commercial Brands of Yeast
Revista de Microbiologia (1999) 30:253-257 Killer toxin of S. cerevisiae ISSN 0001-3714 KILLER TOXIN OF SACCHAROMYCES CEREVISIAE Y500-4L ACTIVE AGAINST FLEISCHMANN AND ITAIQUARA COMMERCIAL BRANDS OF YEAST Giselle A.M. Soares*; Hélia H. Sato Departamento de Ciência de Alimentos, Faculdade de Engenharia de Alimentos, Universidade Estadual de Campinas, Campinas, SP, Brasil Submitted: October 02, 1998; Returned to authors for corrections: March 03, 1999; Approved: May 11, 1999 ABSTRACT The strain Saccharomyces cerevisiae Y500-4L, previously selected from the must of alcohol producing plants and showing high fermentative and killer capacities, was characterized according to the interactions between the yeasts and examined for curing and detection of dsRNA plasmids, which code for the killer character. The killer yeast S. cerevisiae Y500-4L showed considerable killer activity against the Fleischmann and Itaiquara commercial brands of yeast and also against the standard killer yeasts K2 (S. diastaticus NCYC 713), K4 (Candida glabrata NCYC 388) and K11 (Torulopsis glabrata ATCC 15126). However S. cerevisiae Y500-4L showed sensitivity to the killer toxin produced by the standard killer yeasts K8 (Hansenula anomala NCYC 435), K9 (Hansenula mrakii NCYC 500), K10 (Kluyveromyces drosophilarum NCYC 575) and K11 (Torulopsis glabrata ATCC 15126). No M-dsRNA plasmid was detected in the S. cerevisiae Y500-4L strain and these results suggest that the genetic basis for toxin production is encoded by chromosomal DNA. The strain S. cerevisiae Y500-4L was more resistant to the loss of the phenotype killer with cycloheximide and incubation at elevated temperatures (40oC) than the standard killer yeast S. cerevisiae K1. -

A Nucleic Acid Associated with a Killer Strain of Yeast (Double-Stranded RNA/Cesium Sulfate)
Proc. Nat. Acad. Sci. USA Vol. 70, No. 4, pp. 1069-1072, April 1973 A Nucleic Acid Associated with a Killer Strain of Yeast (double-stranded RNA/cesium sulfate) MICHAEL H. VODKIN AND GERALD R. FINK Section of Genetics, Development, and Physiology, Cornell University, Ithaca, New York 14850 Communicated by Adrian M. Srb, January 26, 1973 ABSTRACT A crude membrane fraction was isolated 1 mCi of [8-'H]adenine was added, and the culture was in- from both killer M(k) and isogenic nonkiller M(o) strains cubated at 300 for 16-18 hr (24-27 hr for p- strain) in a of yeast labeled with [3H]adenine. Nucleic acids were ex- tracted from this fraction and centrifuged to equilibrium gyrotory shaker. in an ethidium bromide-Cs2SO4 solution. A peak of radio- activity coincident with a single fluorescent band was Nucleic Acid Extraction and EtBr-Cs2SO4 Centrifugation. present in the membrane fraction isolated from cells of By the procedure of Clark-Walker (4), a crude membrane the killer strain but was absent from that isolated from fraction was obtained from 25-40 g of yeast broken in a nonkiller cells. This material has been characterized as Braun homogenizer. EtBr at a concentration of 500 ag/ml double-stranded RNA by treatment with various nucleases The and by chromatography on a cellulose CF-li column. was present in all buffers used during the isolation. pellet Sedimentation in a sucrose gradient indicates an S value was lysed in 2.5 ml of 1% Brij 35. To the lysate was added 0.5 of 8-10. -

Scent of a Killer: How Could Killer Yeast Boost Its Dispersal?
Received: 9 March 2021 | Accepted: 17 March 2021 DOI: 10.1002/ece3.7534 NATURE NOTES Scent of a killer: How could killer yeast boost its dispersal? Claudia C. Buser1,2 | Jukka Jokela1,2 | Oliver Y. Martin1,3 1Institute of Integrative Biology, ETH Zürich, Zürich, Switzerland Abstract 2Department of Aquatic Ecology, Eawag, Vector- borne parasites often manipulate hosts to attract uninfected vectors. For Dübendorf, Switzerland example, parasites causing malaria alter host odor to attract mosquitoes. Here, we 3Department of Biology, ETH Zürich, Zürich, Switzerland discuss the ecology and evolution of fruit- colonizing yeast in a tripartite symbiosis— the so- called “killer yeast” system. “Killer yeast” consists of Saccharomyces cerevi- Correspondence Claudia C. Buser, Dep. Environmental siae yeast hosting two double- stranded RNA viruses (M satellite dsRNAs, L- A dsRNA Sciences, ETH, Überlandstrasse 133, 8309 helper virus). When both dsRNA viruses occur in a yeast cell, the yeast converts to Dübendorf, Switzerland. Email: [email protected] lethal toxin-producing “killer yeast” phenotype that kills uninfected yeasts. Yeasts on ephemeral fruits attract insect vectors to colonize new habitats. As the viruses Funding information ETH Research, Grant/Award Number: ETH- have no extracellular stage, they depend on the same insect vectors as yeast for 23 20- 1; Association for the Study of Animal their dispersal. Viruses also benefit from yeast dispersal as this promotes yeast to Behavior reproduce sexually, which is how viruses can transmit to uninfected yeast strains. We tested whether insect vectors are more attracted to killer yeasts than to non-killer yeasts. In our field experiment, we found that killer yeasts were more attractive to Drosophila than non- killer yeasts. -

Yeasts and Yeast-Like Fungi As an Element of Purity Assessment of Surface Waters
Polish J. of Environ. Stud. Vol. 20, No. 2 (2011), 267-274 Original Research Yeasts and Yeast-Like Fungi as an Element of Purity Assessment of Surface Waters Anna Biedunkiewicz*, Ewelina Baranowska Chair of Mycology, University of Warmia and Mazury, Oczapowskiego 1A, 10-957 Olsztyn, Poland Received: 2 August 2010 Accepted: 9 November 2010 Abstract The aim of our study was to determine species biodiversity of yeasts and yeast-like fungi in the stratum of surface water of Lake Tyrsko, as well as to evaluate the condition of waters of that lake based on the iso- lated species of fungi. The study was carried out from June 2007 to June 2008. The isolation and determination of the number of fungal colonies were conducted with the use of the membrane filter method. Fungal diagnostics were con- ducted based on the morphological and biochemical characteristics of the fungi. These characteristics were additionally used for taxonomic identification. In the course of the study we isolated 56 species of yeasts and yeast-like fungi belonging to 26 genera, with the predominating genus being Kluyveromyces (13 species), followed by less frequent genera: Candida (8 species), and Debaryomyces (7 species). The highest number of isolates were obtained in the spring (51), fewer in the summer (36), and the lowest in autumn (15). Species indicatory of the self-purifying process of the water examined were frequently isolated (Debaryomyces hansenii, Pichia membranifaciens). Results obtained in the study confirm suggestions of other authors that yeasts and yeast-like fungi may be useful as indicatory organisms showing the extent and character of water contamination and as bioindicators of sanitary and hygienic evaluation of water. -

Protease B of the Lysosomelike Vacuole of the Yeast Saccharomyces Cerevisiae Is Homologous to the Subtilisin Family of Serine Proteases CHARLES M
MOLECULAR AND CELLULAR BIOLOGY, Dec. 1987, p. 4390-4399 Vol. 7, No. 12 0270-7306/87/124390-10$02.00/0 Copyright © 1987, American Society for Microbiology Protease B of the Lysosomelike Vacuole of the Yeast Saccharomyces cerevisiae Is Homologous to the Subtilisin Family of Serine Proteases CHARLES M. MOEHLE,1* RICHARD TIZARD,2 SANDRA K. LEMMON,1 JOHN SMART,2 AND ELIZABETH W. JONES1 Department ofBiological Sciences, Carnegie Mellon University, Pittsburgh, Pennsylvania 15213,1 and Biogen, Cambridge, Massachusetts 021422 Received 22 May 1987/Accepted 2 September 1987 The PRB1 gene of Saccharomyces cerevisiae encodes the vacuolar endoprotease protease B. We have determined the DNA sequence of the PRBI gene and the amino acid sequence of the amino terminus of mature protease B. The deduced amino acid sequence of this serine protease shares extensive homology with those of subtilisin, proteinase K, and related proteases. The open reading frame of PRB1 consists of 635 codons and, therefore, encodes a very large protein (molecular weight, >69,000) relative to the observed size of mature protease B (molecular weight, 33,000). Examination of the gene sequence, the determined amino-terminal sequence, and empirical molecular weight determinations suggests that the preproenzyme must be processed at both amino and carboxy termini and that asparagine-linked glycosylation occurs at an unusual tripeptide acceptor sequence. The lysosomelike vacuole of the yeast Saccharomyces carbohydrate is not Asn linked and may be linked to cerevisiae contains a number of the major hydrolases of the hydroxyl groups (see reference 5 for a review). cell, including protease A, protease B, carboxypeptidase Y, We have recently isolated the structural gene for protease the large aminopeptidase, repressible alkaline phosphatase, B, PRBI, and have described its transcription pattern (47). -

In Killer Strains of Saccharomyces Cerevisiae
TWO CHROMOSOMAL GENES REQUIRED FOR KILLING EXPRESSION IN KILLER STRAINS OF SACCHAROMYCES CEREVISIAE REED B. WICKNER AND MICHAEL J. LEIBOWITZ’ Laboratory of Biochemical Pharmacology, National Institute of Arthritis, Metabolism, and Digestive Diseases, National Institutes of Health, Bethesda, Maryland 20014 Manuscript received September 15, 1975 ABSTRACT The killer character of yeast is determined by a 1.4 x 106 molecular weight double-stranded RNA plasmid and at least 12 chromosomal genes. Wild-type strains of yeast that carry this plasmid (killers) secrete a toxin which is lethal only to strains not carrying this plasmid (sensitives). -__ We have isolated 28 independent recessive chromosomal mutants of a killer strain that have lost the ability to secrete an active toxin but remain resistant to the effects of the toxin and continue to carry the complete cytoplasmic killer genome. These mutants define two complementation groups, ked and kex2. Kexl is located on chromosome VIZ between ade5 and lys5. Kex2 is located on chromosome XIV, but it does not show meiotic linkage to any gene previously located on this chromosome. __ When the killer plasmid of kexl or kex2 strains is elimi- nated by curing with heat or cycloheximide, the strains become sensitive to killing. The mutant phenotype reappears among the meiotic segregants in a cross with a normal killer. Thus, the kex phenotype does not require an alteration of the killer plasmid. --- Kexl and kex2 strains each contain near-normal levels of the 1.4 x 106 molecular weight double-stranded RNA, whose presence is correlated with the presence of the killer genome. CERTAIN strains of Saccharomyces cereuisiae (called killers) secrete a pro- tein which kills other strains (called sensitives) (MAKOWERand BEVAN 1963; WOODSand BEVAN1968; BUSSEY1972). -

The Classification of Orphans Is Improved by Combining Searches in Both Proteomes and Genomes
bioRxiv preprint doi: https://doi.org/10.1101/185983; this version posted May 19, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. The classification of orphans is improved by combining searches in both proteomes and genomes. Walter Basile1,2, Marco Salvatore1,2, Arne Elofsson1,2,3,*, 1 Science for Life Laboratory, Stockholm University SE-171 21 Solna, Sweden 2 Department of Biochemistry and Biophysics, Stockholm University, SE-106 91 Stockholm, Sweden 3 Swedish e-Science Research Center (SeRC) * Corresponding author: [email protected] Abstract The identification of de novo created genes is important as it provides a glimpse on the evolutionary processes of gene creation. Potential de novo created genes are identified by selecting genes that have no homologs outside a particular species, but for an accurate detection this identification needs to be correct. Genes without any homologs are often referred to as orphans; in addition to de novo created ones, fast evolving genes or genes lost in all related genomes might also be classified as orphans. The identification of orphans is dependent on: (i) a method to detect homologs and (ii) a database including genes from related genomes. Here, we set out to investigate how the detection of orphans is influenced by these two factors. Using Saccharomyces cerevisiae we identify that best strategy is to use a combination of searching annotated proteins and a six-frame translation of all ORFs from closely related genomes. -

Comparative Genomics of Protoploid Saccharomycetaceae
Downloaded from genome.cshlp.org on October 5, 2021 - Published by Cold Spring Harbor Laboratory Press Evolution of protoploid yeast genomes ___________________________________________________________________________ Comparative genomics of protoploid Saccharomycetaceae. The Génolevures Consortium (1) Running title: Evolution of protoploid yeast genomes Key words: protein families, synteny, tandems, annotation, SONS, ancestor genome Corresponding author: Jean Luc Souciet Université de Strasbourg, CNRS, UMR 7156 Institut de Botanique, 28 rue Goethe, F-67000 Strasbourg, France Tel: 33 3 90 24 18 17 FAX: 33 3 90 24 20 28 e-mail: [email protected] (1) List of participants and affiliations appear at the end of the paper 1 Downloaded from genome.cshlp.org on October 5, 2021 - Published by Cold Spring Harbor Laboratory Press Evolution of protoploid yeast genomes ___________________________________________________________________________ Abstract Our knowledge on yeast genomes remains largely dominated by the extensive studies on Saccharomyces cerevisiae and the consequences of its ancestral duplication, leaving the evolution of the entire class of hemiascomycetes only partly explored. We concentrate here on five species of Saccharomycetaceae, a large subdivision of hemiascomycetes, that we call “protoploid” because they diverged from the S. cerevisiae lineage prior to its genome duplication. We determined the complete genome sequences of three of these species, Kluyveromyces (Lachancea) thermotolerans and Saccharomyces (Lachancea) kluyveri (two members of the newly described Lachancea clade) and Zygosaccharomyces rouxii. We included in our comparisons the previously available sequences of Klyveromyces lactis and Ashbya (Eremothecium) gossypii. Despite their broad evolutionary range and significant individual variations in each lineage, the five protoploid Saccharomycetaceae share a core repertoire of ca. -

Dissertation, Bayreuth 2003
Molekularsystematische Untersuchungen an Vertretern der pflanzenparasitischen Gattung Exobasidium (Basidiomycota) Dissertation zur Erlangung des Grades eines Doktors der Naturwissenschaften - Dr. rer. nat. - an der Fakultät für Biologie/ Chemie/ Geowissenschaften der Universität Bayreuth vorgelegt von Diplom-Biologin Heidi Döring aus Kassel Bayreuth 2003 Die vorliegende Arbeit wurde am Lehrstuhl für Pflanzensystematik der Universität Bayreuth unter der Betreuung von Herrn Prof. Dr. P. BLANZ (jetzt: Karl-Franzens-Universität Graz) angefertigt. Die dieser Arbeit zugrunde liegenden praktischen Laborarbeiten wurden im Zeitraum von Juli 1992 bis Januar 1995 sowie von März bis August 1997 durchgeführt. Teile der Arbeit wurden durch ein Stipendium und Forschungsgelder des Graduiertenkollegs „Pflanzen-Herbivoren-Systeme“ der Universität Bayreuth gefördert. Vollständiger Abdruck der von der Fakultät für Biologie, Chemie und Geowissenschaften der Universität Bayreuth genehmigten Dissertation zur Erlangung des akademischen Grades Doktor der Naturwissenschaften (Dr. rer. nat.). Tag der Abgabe: 18.12.2003 Tag des wissenschaftlichen Kolloquiums: 07.05.2004 Mitglieder des Prüfungsausschusses: Herr Prof. Dr. P. BLANZ (Gutachter) Herr Prof. Dr. G. KRAUSS (Vorsitzender) Frau Prof. Dr. S. LIEDE-SCHUMANN Herr Prof. Dr. G. RAMBOLD (Gutachter) Herr Prof. Dr. W. SCHUMANN Teilergebnisse dieser Arbeit sind bereits veröffentlicht: BLANZ, P. & H. DÖRING (1995) Taxonomic relationships in the genus Exobasidium (Basidio- mycetes) based on ribosomal DNA analysis. Studies in Mycology 38: 119-127. DÖRING, H. & P. BLANZ (2000) 18S rDNA-Analysen bei der Gattung Exobasidium. Hoppea 61: 85-100. Teilergebnisse dieser Arbeit wurden auf Tagungen vorgestellt: Vorträge DÖRING, H. „RFLP-Analysen bei Arten der Gattung Exobasidium“- IV. Tagung der Gesell- schaft für Mykologie und Lichenologie, 16.-18.3.1994, Braunschweig. DÖRING, H., R. -

Global Expression Analysis of the Yeast Lachancea
Washington University School of Medicine Digital Commons@Becker Open Access Publications 11-2013 Global expression analysis of the yeast Lachancea (saccharomyces) kluyveri reveals new URC genes involved in pyrimidine catabolism Anna Andersson Rasmussen Lund University Dineshkumar Kandasamy Lund University Halfdan Beck Lund University Seth D. Crosby Washington University School of Medicine in St. Louis Olof Bjornberg Lund University See next page for additional authors Follow this and additional works at: https://digitalcommons.wustl.edu/open_access_pubs Recommended Citation Rasmussen, Anna Andersson; Kandasamy, Dineshkumar; Beck, Halfdan; Crosby, Seth D.; Bjornberg, Olof; Schnackerz, Klaus D.; and Piskur, Jure, ,"Global expression analysis of the yeast Lachancea (saccharomyces) kluyveri reveals new URC genes involved in pyrimidine catabolism." Eukaryotic Cell.13,1. 31-42. (2013). https://digitalcommons.wustl.edu/open_access_pubs/2116 This Open Access Publication is brought to you for free and open access by Digital Commons@Becker. It has been accepted for inclusion in Open Access Publications by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. Authors Anna Andersson Rasmussen, Dineshkumar Kandasamy, Halfdan Beck, Seth D. Crosby, Olof Bjornberg, Klaus D. Schnackerz, and Jure Piskur This open access publication is available at Digital Commons@Becker: https://digitalcommons.wustl.edu/open_access_pubs/2116 Global Expression Analysis of the Yeast Lachancea (Saccharomyces) kluyveri Reveals New URC Genes Involved in Pyrimidine Catabolism Anna Andersson Rasmussen, Dineshkumar Kandasamy, Downloaded from Halfdan Beck, Seth D. Crosby, Olof Björnberg, Klaus D. Schnackerz and Jure Piskur Eukaryotic Cell 2014, 13(1):31. DOI: 10.1128/EC.00202-13. Published Ahead of Print 1 November 2013. http://ec.asm.org/ Updated information and services can be found at: http://ec.asm.org/content/13/1/31 These include: SUPPLEMENTAL MATERIAL Supplemental material on January 28, 2014 by Washington University in St. -

Conservation of Mrna Quality Control Factor Ski7 and Its Diversification Through Changes in Alternative Splicing and Gene Duplication
Conservation of mRNA quality control factor Ski7 and its diversification through changes in alternative splicing and gene duplication Alexandra N. Marshalla, Jaeil Hana, Minseon Kima, and Ambro van Hoofa,1 aDepartment of Microbiology and Molecular Genetics, University of Texas Health Science Center at Houston and University of Texas Graduate School of Biomedical Sciences, Houston, TX 77030 Edited by Lynne E. Maquat, University of Rochester School of Medicine and Dentistry, Rochester, NY, and approved May 7, 2018 (received for review February 2, 2018) Eukaryotes maintain fidelity of gene expression by preferential Ski7 function was initially thought to have arisen after the du- degradation of aberrant mRNAs that arise by errors in RNA plication, we have previously shown that the single Lachancea processing reactions. In Saccharomyces cerevisiae, Ski7 plays an kluyveri ortholog can carry out both Ski7 and Hbs1 functions and important role in this mRNA quality control by mediating mRNA thus, that Ski7 function was already present before whole ge- degradation by the RNA exosome. Ski7 was initially thought to be nome duplication (8, 10, 11). In addition, we have shown that the restricted to Saccharomyces cerevisiae and close relatives because L. kluyveri gene encodes two different proteins through alter- the SKI7 gene and its paralog HBS1 arose by whole genome du- native splicing with a shorter splice isoform performing the plication (WGD) in a recent ancestor. We have recently shown that function of HBS1 and a longer splice isoform performing the the preduplication gene was alternatively spliced and that Ski7 function of SKI7 (10). Furthermore, based on sequence analysis of function predates WGD. -
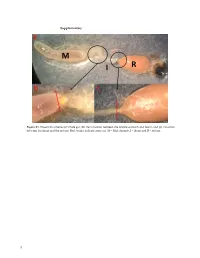
Supplementary File 1
Supplementary Figure S1. Dissection scheme (a) whole gut, (b) the transition between the middle stomach and ileum, and (c) transition between the ileum and the rectum. Red streaks indicate areas cut. M = Mid stomach, I = ileum and R = rectum. 1 Figure S2. Relative abundance of the 20 most abundant fungal OTUs across months per gut part. Taxonomic assignments are provided in Table S1. 2 Figure S3. Relative abundance of the bacterial OUTs (with abundance > 1%) across months per gut part. The color code is given at the genus level. 3 Table S1. Taxonomic assignments of fungal OTUs by Blast. OTU# Taxonomy by blast nt collection 11/11‐2020 Phylum Accesion# OTU_1 Aureobasidium pullulans Ascomycota MW085051 OTU_2 Meyerozyma guilliermondii Ascomycota MT988167 OTU_3 Starmerella apicola Ascomycota KY101940 OTU_4 Debaryomyces hansenii Ascomycota MW051606 OTU_5 Engyodontium sp. Ascomycota MN905797 Basidiomy‐ OTU_6 Mrakia sp. MT505696 cota Basidiomy‐ OTU_7 Tausonia pullulans MN900123 cota OTU_8 Unknown OTU_9 Unknown OTU_10 Penicillium corylophilum Ascomycota MT906500 OTU_11 Penicillium sp. Ascomycota MT993349 OTU_12 Cladosporium sp. Ascomycota MW077705 Phaffia rhodozyma/Xanthophyllomyces dendro‐ Basidiomy‐ OTU_13 KY104501/DQ904243 rhous cota Basidiomy‐ OTU_14 Filobasidium wieringae MN899199 cota OTU_15 Hanseniaspora uvarum Ascomycota MN556596 Basidiomy‐ OTU_16 Mrakia gelida MN460370 cota OTU_17 Uncultured fungus clone MK717974 OTU_18 Cladosporium allicinum Ascomycota MT974153 OTU_20 Taphrina carpini Ascomycota MK782181 Basidiomy‐ OTU_25 Papiliotrema