Environmental Protection Agency § 117.3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(A) 1) at First a Pair of Each 10G. of Sample Was Extracted with Ether by the Soxhlet's Apparatus for a Week
52 [vol. 6, THE DISTRIBUTION OF PHOSPHORUS IN COW'S MILK AND THE SCHEME FOR THE SEPARATION OF PHOSPHATIDES By Rinjiro SASAKI (From the Institute of Agricultural Chemistry, College of Agriculture, Tokyo Imperial University) (Received 9th September 1930) A. The Distribution of Phosphorus in Cow's milk In order to extract the phosphatides, it is necessary first to determine the distribution of phosphorus in cow's milk. Liquid milk can not be extracted with ether or with other organic solvents owing to its large content of water. The substance applied in this experiment is the milk powder, dried by the Buflovak drum drier below 70•Ž. Its content of moisture is 4.498%, deter mined by the method of drying in the air bath of 105•Ž. The content of total phosphorus was determined(5) in the ash of sample which was burned with a little of fusing mixture. In 100g. of milk powder In 100g. of dry matter Total phosphorus 0.745g. 0.781g. The amounts of phosphorus, which are soluble in the various organic sol vents, were determined by extracting with three solvents in the following orders. (A) Ether-Acetone-Alcohol (B) Acetone-Ether-Alcohol (C) Alcohol-Ether-Acetone Experiment (A) 1) At first a pair of each 10g. of sample was extracted with ether by the Soxhlet's apparatus for a week. After the extraction had been comp leted, the extract was freed from ether and weighed. In 100g.of milkpowder In 100g.of dry matter Total ether matters soluble 3.609g. 3.779g. 2) The above extract was dissolved in a very little of ether. -

PRICELIST-1920-FINAL.Pdf
INDEX Page No. MD Speech 01 Our Vision / Our Mission 02 Product Classification and Grade Information 03 Label Information 04 GHS Compliance 05 Technical Data Sheet and COA 06 Qualikems Product Range 07 ISO Certificate 08 - 09 Company Details 10 Ordering Information 11 Terms & Conditions 12 Rate List 13 - 52 Images of Lab / Plant / R & D 53 - 58 Rate List 59 -116 BELIEVING yourselfIN IS THE FIRST SECRET TO Success Dear Reader, The document you are holding is the result of work performed by the team of professionals of QUALIKEMS. It is the fruit of our teams extensive technical experience combine with the collaboration of our customers, who have offered us their valuable comments and proposals for improvement. At Qualikems, we have been working and investing for many years with our thoughts focused on the long term. Only thus can this comprehensive catalogue be kept up to date with the products you need. Our highly trained workforce, using state of the art technology, is the driving force behind the management of our modern factory, and our principal aim is to guarantee that the QUALIKEMS product range meets the conditions you require. QUALIKEMS reinforces industrial character and the path to progress we have continuously forged over the years. This path requires the responsible use of resources and the sustainability of our business activity. It is likewise requires and ability to keep on growing as the way to earn and to preserve our status as the leading supplier of laboratory reagents to our Clients Ashok Sahni Managing Director QUALIKEMS FINE CHEM PVT. -

162 Part 175—Indirect Food Addi
§ 174.6 21 CFR Ch. I (4–1–19 Edition) (c) The existence in this subchapter B Subpart B—Substances for Use Only as of a regulation prescribing safe condi- Components of Adhesives tions for the use of a substance as an Sec. article or component of articles that 175.105 Adhesives. contact food shall not be construed as 175.125 Pressure-sensitive adhesives. implying that such substance may be safely used as a direct additive in food. Subpart C—Substances for Use as (d) Substances that under conditions Components of Coatings of good manufacturing practice may be 175.210 Acrylate ester copolymer coating. safely used as components of articles 175.230 Hot-melt strippable food coatings. that contact food include the fol- 175.250 Paraffin (synthetic). lowing, subject to any prescribed limi- 175.260 Partial phosphoric acid esters of pol- yester resins. tations: 175.270 Poly(vinyl fluoride) resins. (1) Substances generally recognized 175.300 Resinous and polymeric coatings. as safe in or on food. 175.320 Resinous and polymeric coatings for (2) Substances generally recognized polyolefin films. as safe for their intended use in food 175.350 Vinyl acetate/crotonic acid copoly- mer. packaging. 175.360 Vinylidene chloride copolymer coat- (3) Substances used in accordance ings for nylon film. with a prior sanction or approval. 175.365 Vinylidene chloride copolymer coat- (4) Substances permitted for use by ings for polycarbonate film. 175.380 Xylene-formaldehyde resins con- regulations in this part and parts 175, densed with 4,4′-isopropylidenediphenol- 176, 177, 178 and § 179.45 of this chapter. -

Environmental Protection Agency § 117.3
Environmental Protection Agency § 117.3 (iii) Which are used or could be used (j) Process waste water means any for industrial purposes by industries in water which, during manufacturing or interstate commerce; processing, comes into direct contact (4) All impoundments of waters oth- with or results from the production or erwise defined as navigable waters use of any raw material, intermediate under this paragraph; product, finished product, byproduct, (5) Tributaries of waters identified in or waste product. paragraphs (i) (1) through (4) of this section, including adjacent wetlands; [44 FR 50776, Aug. 29, 1979, as amended at 58 and FR 45039, Aug. 25, 1993; 65 FR 30904, May 15, (6) Wetlands adjacent to waters iden- 2000] tified in paragraphs (i) (1) through (5) of this section (‘‘Wetlands’’ means § 117.2 Abbreviations. those areas that are inundated or satu- NPDES equals National Pollutant rated by surface or ground water at a Discharge Elimination System. RQ frequency and duration sufficient to equals reportable quantity. support, and that under normal cir- cumstances do support, a prevalence of § 117.3 Determination of reportable vegetation typically adapted for life in quantities. saturated soil conditions. Wetlands Each substance in Table 117.3 that is generally included playa lakes, listed in Table 302.4, 40 CFR part 302, is swamps, marshes, bogs, and similar assigned the reportable quantity listed areas such as sloughs, prairie potholes, in Table 302.4 for that substance. wet meadows, prairie river overflows, mudflats, and natural ponds): Provided, TABLE 117.3—REPORTABLE QUANTITIES That waste treatment systems (other OF HAZARDOUS SUBSTANCES DES- than cooling ponds meeting the cri- IGNATED PURSUANT TO SECTION 311 OF teria of this paragraph) are not waters THE CLEAN WATER ACT of the United States. -

United States Patent Office Patented Apr
3,030,421 United States Patent Office Patented Apr. 17, 1962 2 3,030,421 perature and under a reduced pressure. In the case of PROCESS FOR PREPARNG TRHYDROXY most of the catalysts the reaction products, which are free METHYL-PHOSPHINE s from solvents, solidify already when being cooled to room Martin Reuter and Ludwig Ortane, Frankfurt an Main, temperature. If, in the case of some catalysts, they Germany, assignors to Farbwerke Hoechst Aktienger 5 solidify only at a lower temperature and still contain to sellschaft vormas Meister Lucius & Briining, Frank a greater extent oily by-products and/or phosphonium furt am Main, Germany, a corporation of Gerinally hydroxide, they can be separated from the latter by filter No Drawing. Fied Jaa. 14, 1958, Ser. No. 708,764 ing or pressing. - - - Claims priority, application Germany Jan. 23, 1957 It is to be assumed that the crystalline main product 6 Canas. (C. 260-606.5) O of the present invention constitutes the hitherto unknown We have found that a new and valuable phosphorus trihydroxymethyl-phosphine. Main and by-products are compound carrying hydroxymethyl groups at the phos easily soluble in water and methanol and sparingly solu phorus atom can be prepared by reacting 1 mol of formal ble in fat dissolvers. dehyde with 4 mol of phosphine, preferably in the pres The reaction products of the invention can be used as ence of water, and in the presence of Small quantities of 5 insecticides, additives for lubricants, flame-proofing agents finely distributed metals that do not belong to the alkali for wood and textiles and as intermediates for these sub metals or alkaline earth metals and/or their compounds Staces. -

Refrigerant Safety Refrigerant History
Refrigerant Safety The risks associated with the use of refrigerants in refrigeration and airconditioning equipment can include toxicity, flammability, asphyxiation, and physical hazards. Although refrigerants can pose one or more of these risks, system design, engineering controls, and other techniques mitigate this risk for the use of refrigerant in various types of equipment. Refrigerant History Nearly all of the historically used refrigerants were flammable, toxic, or both. Some were also highly reactive, resulting in accidents (e. g., leak, explosion) due to equipment failure, poor maintenance, or human error. The task of finding a nonflammable refrigerant with good stability was given to Thomas Midgley in 1926. With his associates Albert Leon Henne and Robert Reed McNary, Dr. Midgley observed that the refrigerants then in use comprised relatively few chemical elements, many of which were clustered in an intersecting row and column of the periodic table of elements. The element at the intersection was fluorine, known to be toxic by itself. Midgley and his collaborators felt, however, that compounds containing fluorine could be both nontoxic and nonflammable. The attention of Midgley and his associates was drawn to organic fluorides by an error in the literature that showed the boiling point for tetrafluoromethane (carbon tetrafluoride) to be high compared to those for other fluorinated compounds. The correct boiling temperature later was found to be much lower. Nevertheless, the incorrect value was in the range sought and led to evaluation of organic fluorides as candidates. The shorthand convention, later introduced to simplify identification of the organic fluorides for a systematic search, is used today as the numbering system for refrigerants. -

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

Environmental Protection Agency § 117.3
Environmental Protection Agency § 117.3 (4) Applicability date. This paragraph TABLE 117.3—REPORTABLE QUANTITIES OF (i) is applicable beginning on February HAZARDOUS SUBSTANCES DESIGNATED PUR- 6, 2020. SUANT TO SECTION 311 OF THE CLEAN (j) Process waste water means any WATER ACT—Continued water which, during manufacturing or Cat- RQ in pounds processing, comes into direct contact Material egory (kilograms) with or results from the production or use of any raw material, intermediate Ammonium benzoate ...................... D ...... 5,000 (2,270) Ammonium bicarbonate .................. D ...... 5,000 (2,270) product, finished product, byproduct, Ammonium bichromate ................... A ....... 10 (4.54) or waste product. Ammonium bifluoride ...................... B ....... 100 (45.4) Ammonium bisulfite ......................... D ...... 5,000 (2,270) [44 FR 50776, Aug. 29, 1979, as amended at 58 Ammonium carbamate .................... D ...... 5,000 (2,270) FR 45039, Aug. 25, 1993; 65 FR 30904, May 15, Ammonium carbonate ..................... D ...... 5,000 (2,270) 2000; 80 FR 37112, June 29, 2015; 83 FR 5208, Ammonium chloride ........................ D ...... 5,000 (2,270) Feb. 6, 2018] Ammonium chromate ...................... A ....... 10 (4.54) Ammonium citrate dibasic ............... D ...... 5,000 (2,270) Ammonium fluoborate ..................... D ...... 5,000 (2,270) § 117.2 Abbreviations. Ammonium fluoride ......................... B ....... 100 (45.4) NPDES equals National Pollutant Ammonium hydroxide ..................... C -

Thermodynamic Properties of Mixtures of Aqueous Solutions of Hydrochloric Acid and Cadmium Chloride, Copper Chloride, Manganese Chloride, and Zinc Chloride
Western Michigan University ScholarWorks at WMU Master's Theses Graduate College 12-1993 Thermodynamic Properties of Mixtures of Aqueous Solutions of Hydrochloric Acid and Cadmium Chloride, Copper Chloride, Manganese Chloride, and Zinc Chloride Samia A. Kosa Follow this and additional works at: https://scholarworks.wmich.edu/masters_theses Part of the Chemistry Commons Recommended Citation Kosa, Samia A., "Thermodynamic Properties of Mixtures of Aqueous Solutions of Hydrochloric Acid and Cadmium Chloride, Copper Chloride, Manganese Chloride, and Zinc Chloride" (1993). Master's Theses. 4363. https://scholarworks.wmich.edu/masters_theses/4363 This Masters Thesis-Open Access is brought to you for free and open access by the Graduate College at ScholarWorks at WMU. It has been accepted for inclusion in Master's Theses by an authorized administrator of ScholarWorks at WMU. For more information, please contact [email protected]. THERMODYNAMIC PROPERTIES OF MIXTURES OF AQUEOUS SOLUTIONS OF HYDROCHLORIC ACID AND CADMIUM CHLORIDE, COPPER CHLORIDE, MANGANESE CHLORIDE, AND ZINC CHLORIDE by Samia A. Kosa A Thesis Submitted to the Faculty of The Graduate College in partial fulfillment of the requirements for the Degree of Master of Arts Department of Chemistry Western Michigan University Kalamazoo, Michigan December 1993 ACKNOWLEDGEMENTS I wish to express my deep gratitude and sincere appreciation to my committee advisor, Dr. Donald Schriber, for his continual guidance, direction, and assistance in this research. I would also like to acknowledge the help of the other committee members, Dr. Thomass Houser and Dr. Ralph Steinhaus, towards this work. My family continuously provided me support and encouragement and to them I owe more than I can say. -

Used at Rocky Flats
. TASK 1 REPORT (Rl) IDENTIFICATION OF CHEMICALS AND RADIONUCLIDES USED AT ROCKY FLATS I PROJECT BACKGROUND ChemRisk is conducting a Rocky Flats Toxicologic Review and Dose Reconstruction study for The Colorado Department of Health. The two year study will be completed by the fall of 1992. The ChemRisk study is composed of twelve tasks that represent the first phase of an independent investigation of off-site health risks associated with the operation of the Rocky Flats nuclear weapons plant northwest of Denver. The first eight tasks address the collection of historic information on operations and releases and a detailed dose reconstruction analysis. Tasks 9 through 12 address the compilation of information and communication of the results of the study. Task 1 will involve the creation of an inventory of chemicals and radionuclides that have been present at Rocky Flats. Using this inventory, chemicals and radionuclides of concern will be selected under Task 2, based on such factors as the relative toxicity of the materials, quantities used, how the materials might have been released into the environment, and the likelihood for transport of the materials off-site. An historical activities profile of the plant will be constructed under Task 3. Tasks 4, 5, and 6 will address the identification of where in the facility activities took place, how much of the materials of concern were released to the environment, and where these materials went after the releases. Task 7 addresses historic land-use in the vicinity of the plant and the location of off-site populations potentially affected by releases from Rocky Flats. -

123. Antimony
1998:11 The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals 123. Antimony John Erik Berg Knut Skyberg Nordic Council of Ministers arbete och hälsa vetenskaplig skriftserie ISBN 91–7045–471–x ISSN 0346–7821 http://www.niwl.se/ah/ah.htm National Institute for Working Life National Institute for Working Life The National Institute for Working Life is Sweden's center for research and development on labour market, working life and work environment. Diffusion of infor- mation, training and teaching, local development and international collaboration are other important issues for the Institute. The R&D competence will be found in the following areas: Labour market and labour legislation, work organization and production technology, psychosocial working conditions, occupational medicine, allergy, effects on the nervous system, ergonomics, work environment technology and musculoskeletal disorders, chemical hazards and toxicology. A total of about 470 people work at the Institute, around 370 with research and development. The Institute’s staff includes 32 professors and in total 122 persons with a postdoctoral degree. The National Institute for Working Life has a large international collaboration in R&D, including a number of projects within the EC Framework Programme for Research and Technology Development. ARBETE OCH HÄLSA Redaktör: Anders Kjellberg Redaktionskommitté: Anders Colmsjö och Ewa Wigaeus Hjelm © Arbetslivsinstitutet & författarna 1998 Arbetslivsinstitutet, 171 84 Solna, Sverige ISBN 91–7045–471–X ISSN 0346-7821 Tryckt hos CM Gruppen Preface The Nordic Council is an intergovernmental collaborative body for the five countries, Denmark, Finland, Iceland, Norway and Sweden. One of the committees, the Nordic Senior Executive Committee for Occupational Environmental Matters, initiated a project in order to produce criteria documents to be used by the regulatory authorities in the Nordic countries as a scientific basis for the setting of national occupational exposure limits. -
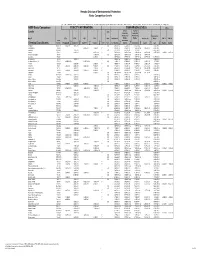
Basic Comparison Levels
Nevada Division of Environmental Protection Basic Comparison Levels Key: I=IRIS; P= PPRTV; N=NCEA; H=HEAST; A=ATSDR; O=Other Documents; CA=CalEPA S=Surrogate X=Appendix PPRTV E=Based on TEF scheme r=Route Extra Key: C = Cancer endpoint; N = Noncancer endpoint; sat = Saturation Limit; max = Ceiling Limit NDEP Basic Comparison TOXICITY INFORMATION COMPARISON LEVELS LBCLs Indoor Outdoor Levels Skin Industrial/ Industrial/ Commercial Commercial Residential May-17 SFo RfDo IUR RfCi Abs. Residential Worker Worker Ambient Air Water DAF 1 DAF 20 CAS w/o Dermal Chemical Constituents Number 1/(mg/kg-d) (mg/kg-d) (ug/m3)-1 (mg/m3) VOCc Soils Soil (mg/kg) (mg/kg) Soil (mg/kg) (µg/m3) (µg/l) (mg/kg) (mg/kg) Key Key key Key Key Key Key Key Key Acephate 30560-19-1 8.70E-03 I 4.00E-03 I 0.10 5.59E+01 C 7.52E+02 C 2.95E+02 C 7.73E+00 C Acetaldehyde 75-07-0 2.20E-06 I 9.00E-03 I V 1.23E+01 C 5.35E+01 C 1.00E+05 max 1.28E+00 C 2.55E+00 C Acetochlor 34256-82-1 2.00E-02 I 0.10 1.23E+03 N 4.67E+04 N 1.83E+04 N 6.67E+02 N Acetone 67-64-1 9.00E-01 I 3.10E+01 A V 7.04E+04 N 1.00E+05 max 1.00E+05 max 3.23E+04 N 2.05E+04 N 8.00E-01 1.60E+01 Acetone Cyanohydrin 75-86-5 2.00E-03 X 0.10 1.00E+05 max 1.00E+05 max 1.00E+05 max 2.09E+00 N Acetonitrile 75-05-8 6.00E-02 I V 1.00E+05 max 3.75E+03 N 1.00E+05 max 6.26E+01 N 1.25E+02 N Acetophenone 98-86-2 1.00E-01 I V 2.52E+03 sat 2.52E+03 sat 2.52E+03 sat 3.34E+03 N Acetylaminofluorene, 2- 53-96-3 3.80E+00 CA 1.30E-03 CA 0.10 1.28E-01 C 1.72E+00 C 6.75E-01 C 2.16E-03 C 1.77E-02 C Acrolein 107-02-8 5.00E-04 I 2.00E-05 I V