Disorders of Neutrophils
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Neutropenia Fact Sheet
Neutropenia in Barth Syndrome i ii (Chronic, Cyclic or Intermittent) What problems can Neutropenia cause? Neutrophils are the main white blood cell for fighting or preventing bacterial or fungal infections. They may be referred to as polymorphonuclear cells (polys or PMNs), white cells with segmented nuclei (segs), or neutrophils in the complete blood cell count (CBC) report. Immature neutrophils are referred to as bands. When someone is neutropenic (an abnormally low level of neutrophils in the blood), the risk of infection increases. The absolute neutrophil count (ANC) is a measure of the total number of neutrophils present in the blood. When the ANC is less than 1,000, the risk of infection increases. Most infections occur in the ears, skin or throat and to a lesser extent, the chest. These infections can be very serious and may require antibiotics to clear infections. When someone with Barth syndrome is neutropenic his defenses are weakened, he is likely to become seriously ill more quickly than someone with a normal neutrophil count. Tips: • No rectal temperatures as any break in the skin can lead to an infection. • If the individual has a temperature > 100.4° F (38° C) or has infectious symptoms, the primary physician or hematologist should be notified. The individual may need to be seen. • If the individual has a temperature of 100.4° F (38° C) – 100.5° F (38.05° C)> 8 hours or a temperature > 101.5° F (38.61° C), an immediate examination by the physician is warranted. Some or all of the following studies may be ordered: CBC with differential and ANC Urinalysis Blood, urine, and other appropriate cultures C-Reactive Protein Echocardiogram if warranted • The physician may suggest antibiotics (and G-CSF if the ANC is low) for common infections such as otitis media, stomatitis. -

THE PATHOLOGY of BONE MARROW FAILURE Roos Leguit, Jan G Van Den Tweel
THE PATHOLOGY OF BONE MARROW FAILURE Roos Leguit, Jan G van den Tweel To cite this version: Roos Leguit, Jan G van den Tweel. THE PATHOLOGY OF BONE MARROW FAILURE. Histopathology, Wiley, 2010, 57 (5), pp.655. 10.1111/j.1365-2559.2010.03612.x. hal-00599534 HAL Id: hal-00599534 https://hal.archives-ouvertes.fr/hal-00599534 Submitted on 10 Jun 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Histopathology THE PATHOLOGY OF BONE MARROW FAILURE ForJournal: Histopathology Peer Review Manuscript ID: HISTOP-02-10-0090 Manuscript Type: Review Date Submitted by the 08-Feb-2010 Author: Complete List of Authors: Leguit, Roos; UMC utrecht, Pathology van den Tweel, Jan; UMC Utrecht, Pathology bone marrow, histopathology, myelodysplastic syndromes, Keywords: inherited bone marrow failure syndromes, trephine biopsy Published on behalf of the British Division of the International Academy of Pathology Page 1 of 40 Histopathology THE PATHOLOGY OF BONE MARROW FAILURE Roos J Leguit & Jan G van den Tweel University Medical Centre Utrecht Department of Pathology H4.312 Heidelberglaan 100 For Peer Review 3584 CX Utrecht The Netherlands Running title: Pathology of bone marrow failure Keywords: bone marrow, histopathology, myelodysplastic syndromes, inherited bone marrow failure syndromes, trephine biopsy. -

Pediatric Oral Pathology. Soft Tissue and Periodontal Conditions
PEDIATRIC ORAL HEALTH 0031-3955100 $15.00 + .OO PEDIATRIC ORAL PATHOLOGY Soft Tissue and Periodontal Conditions Jayne E. Delaney, DDS, MSD, and Martha Ann Keels, DDS, PhD Parents often are concerned with “lumps and bumps” that appear in the mouths of children. Pediatricians should be able to distinguish the normal clinical appearance of the intraoral tissues in children from gingivitis, periodontal abnormalities, and oral lesions. Recognizing early primary tooth mobility or early primary tooth loss is critical because these dental findings may be indicative of a severe underlying medical illness. Diagnostic criteria and .treatment recommendations are reviewed for many commonly encountered oral conditions. INTRAORAL SOFT-TISSUE ABNORMALITIES Congenital Lesions Ankyloglossia Ankyloglossia, or “tongue-tied,” is a common congenital condition characterized by an abnormally short lingual frenum and the inability to extend the tongue. The frenum may lengthen with growth to produce normal function. If the extent of the ankyloglossia is severe, speech may be affected, mandating speech therapy or surgical correction. If a child is able to extend his or her tongue sufficiently far to moisten the lower lip, then a frenectomy usually is not indicated (Fig. 1). From Private Practice, Waldorf, Maryland (JED); and Department of Pediatrics, Division of Pediatric Dentistry, Duke Children’s Hospital, Duke University Medical Center, Durham, North Carolina (MAK) ~~ ~ ~ ~ ~ ~ ~ PEDIATRIC CLINICS OF NORTH AMERICA VOLUME 47 * NUMBER 5 OCTOBER 2000 1125 1126 DELANEY & KEELS Figure 1. A, Short lingual frenum in a 4-year-old child. B, Child demonstrating the ability to lick his lower lip. Developmental Lesions Geographic Tongue Benign migratory glossitis, or geographic tongue, is a common finding during routine clinical examination of children. -

My Beloved Neutrophil Dr Boxer 2014 Neutropenia Family Conference
The Beloved Neutrophil: Its Function in Health and Disease Stem Cell Multipotent Progenitor Myeloid Lymphoid CMP IL-3, SCF, GM-CSF CLP Committed Progenitor MEP GMP GM-CSF, IL-3, SCF EPO TPO G-CSF M-CSF IL-5 IL-3 SCF RBC Platelet Neutrophil Monocyte/ Basophil B-cells Macrophage Eosinophil T-Cells Mast cell NK cells Mature Cell Dendritic cells PRODUCTION AND KINETICS OF NEUTROPHILS CELLS % CELLS TIME Bone Marrow: Myeloblast 1 7 - 9 Mitotic Promyelocyte 4 Days Myelocyte 16 Maturation/ Metamyelocyte 22 3 – 7 Storage Band 30 Days Seg 21 Vascular: Peripheral Blood Seg 2 6 – 12 hours 3 Marginating Pool Apoptosis and ? Tissue clearance by 0 – 3 macrophages days PHAGOCYTOSIS 1. Mobilization 2. Chemotaxis 3. Recognition (Opsonization) 4. Ingestion 5. Degranulation 6. Peroxidation 7. Killing and Digestion 8. Net formation Adhesion: β 2 Integrins ▪ Heterodimer of a and b chain ▪ Tight adhesion, migration, ingestion, co- stimulation of other PMN responses LFA-1 Mac-1 (CR3) p150,95 a2b2 a CD11a CD11b CD11c CD11d b CD18 CD18 CD18 CD18 Cells All PMN, Dendritic Mac, mono, leukocytes mono/mac, PMN, T cell LGL Ligands ICAMs ICAM-1 C3bi, ICAM-3, C3bi other other Fibrinogen other GRANULOCYTE CHEMOATTRACTANTS Chemoattractants Source Activators Lipids PAF Neutrophils C5a, LPS, FMLP Endothelium LTB4 Neutrophils FMLP, C5a, LPS Chemokines (a) IL-8 Monocytes, endothelium LPS, IL-1, TNF, IL-3 other cells Gro a, b, g Monocytes, endothelium IL-1, TNF other cells NAP-2 Activated platelets Platelet activation Others FMLP Bacteria C5a Activation of complement Other Important Receptors on PMNs ñ Pattern recognition receptors – Detect microbes - Toll receptor family - Mannose receptor - bGlucan receptor – fungal cell walls ñ Cytokine receptors – enhance PMN function - G-CSF, GM-CSF - TNF Receptor ñ Opsonin receptors – trigger phagocytosis - FcgRI, II, III - Complement receptors – ñ Mac1/CR3 (CD11b/CD18) – C3bi ñ CR-1 – C3b, C4b, C3bi, C1q, Mannose binding protein From JG Hirsch, J Exp Med 116:827, 1962, with permission. -

Cells, Tissues and Organs of the Immune System
Immune Cells and Organs Bonnie Hylander, Ph.D. Aug 29, 2014 Dept of Immunology [email protected] Immune system Purpose/function? • First line of defense= epithelial integrity= skin, mucosal surfaces • Defense against pathogens – Inside cells= kill the infected cell (Viruses) – Systemic= kill- Bacteria, Fungi, Parasites • Two phases of response – Handle the acute infection, keep it from spreading – Prevent future infections We didn’t know…. • What triggers innate immunity- • What mediates communication between innate and adaptive immunity- Bruce A. Beutler Jules A. Hoffmann Ralph M. Steinman Jules A. Hoffmann Bruce A. Beutler Ralph M. Steinman 1996 (fruit flies) 1998 (mice) 1973 Discovered receptor proteins that can Discovered dendritic recognize bacteria and other microorganisms cells “the conductors of as they enter the body, and activate the first the immune system”. line of defense in the immune system, known DC’s activate T-cells as innate immunity. The Immune System “Although the lymphoid system consists of various separate tissues and organs, it functions as a single entity. This is mainly because its principal cellular constituents, lymphocytes, are intrinsically mobile and continuously recirculate in large number between the blood and the lymph by way of the secondary lymphoid tissues… where antigens and antigen-presenting cells are selectively localized.” -Masayuki, Nat Rev Immuno. May 2004 Not all who wander are lost….. Tolkien Lord of the Rings …..some are searching Overview of the Immune System Immune System • Cells – Innate response- several cell types – Adaptive (specific) response- lymphocytes • Organs – Primary where lymphocytes develop/mature – Secondary where mature lymphocytes and antigen presenting cells interact to initiate a specific immune response • Circulatory system- blood • Lymphatic system- lymph Cells= Leukocytes= white blood cells Plasma- with anticoagulant Granulocytes Serum- after coagulation 1. -
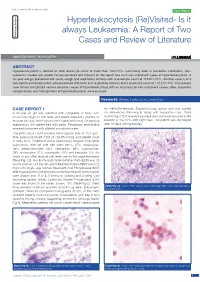
Hyperleukocytosis (Re)Visited- Is It Case Series Always Leukaemia: a Report of Two Pathology Section Cases and Review of Literature Short Communication
Review Article Clinician’s corner Original Article Images in Medicine Experimental Research Miscellaneous Letter to Editor DOI: 10.7860/JCDR/2020/40556.13409 Case Report Postgraduate Education Hyperleukocytosis (Re)Visited- Is it Case Series always Leukaemia: A Report of Two Pathology Section Cases and Review of Literature Short Communication ASHUTOSH RATH1, RICHA GUPTA2 ABSTRACT Hyperleukocytosis is defined as total leukocyte count of more than 100×109/L. Commonly seen in leukaemic conditions, non- leukaemic causes are usually not encountered and thought of. We report two such non-malignant cases of hyperleukocytosis. A six-year old girl presented with fever, cough and respiratory distress with a leukocyte count of 125.97×109/L. Another case is of a two-month old female infant, who presented with fever and respiratory distress and a leukocyte count of 112.27×109/L. The present case thrives to highlight various possible causes of hyperleukocytosis with an emphasis on non-malignant causes. Also, important complications and management of hyperleukocytosis are discussed. Keywords: Benign, Leukocytosis, Leukostasis CASE REPORT 1 for methicillin-resistant Staphylococcus aureus and was started A six-year-old girl was admitted with complaints of fever, non- on intravenous Vancomycin along with supportive care. Serial productive cough for one week and severe respiratory distress for monitoring of TLC revealed a gradual reduction and it returned to the the past one day. There was no other significant history. On physical baseline of 15×109/L after eight days. The patient was discharged examination, the patient had mild pallor. Respiratory examination after 10 days of hospital stay. -

Digitalcommons@UNMC Granulocytopenia
University of Nebraska Medical Center DigitalCommons@UNMC MD Theses Special Collections 5-1-1936 Granulocytopenia Howard E. Mitchell University of Nebraska Medical Center This manuscript is historical in nature and may not reflect current medical research and practice. Search PubMed for current research. Follow this and additional works at: https://digitalcommons.unmc.edu/mdtheses Part of the Medical Education Commons Recommended Citation Mitchell, Howard E., "Granulocytopenia" (1936). MD Theses. 457. https://digitalcommons.unmc.edu/mdtheses/457 This Thesis is brought to you for free and open access by the Special Collections at DigitalCommons@UNMC. It has been accepted for inclusion in MD Theses by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. G PA~lULOCYTOPENI A SENIOR THESIS By Howard E. Mitchell April 17, 1936 TABLE OF CONT'ENTS Introduction Definition • · 1 History . • • • 1 Nomenclature • • • • • 4 ClassificBtion • • • • 6 Physiology • • • • .10 Etiology • • 22 Geographic Distribution • 23 Age, Sex, and R9ce • • ·• 23 Occupation • .. • • • • .. • 23 Ba.cteria • • • • .. 24 Glandu18.r Dysfunction • • • 27 Radiation • • • • 28 Allergy • • • 28 Chemotactic and Maturation Factors • • 28 Chemicals • • • • • 30 Pathology • • • • • 36 Symptoms • • • • • • • 43 DiEtgnosis • • • • • .. • • • • • .. • 4'7 Prognosis 48 '" • • • • • • • • • • • • Treatment • • • • • • • • 49 Non"'specific Therapy • • • • .. 50 Transfusion • • • • .. 51 X-Ray • • • • • • • • • 52 Liver ·Extract • • • • • • • 53 Nucleotides • • • • • • • • • • • 53 General Ca.re • • • • • • • • 57 Conclusion • • • • • • • • • 58 480805 INTHODUCTION Although t~ere is reference in literature of the Nineteenth Century to syndromes similating the disease (granulocytopenia) 9.8 W(~ know it todes, it "vas not un til the year 1922 that Schultz 8ctually described his C8se as a disease entity and by so doing, stimulated the interest of tne medical profession to further in vestigation. -

Chediak Higashi Syndrome Masquerading As Acute Leukemia / Storage Disorder - a Rare Case Report
International Journal of Research in Medical Sciences Asif Baig M et al. Int J Res Med Sci. 2015 Jul;3(7):1785-1787 www.msjonline.org pISSN 2320-6071 | eISSN 2320-6012 DOI: http://dx.doi.org/10.18203/2320-6012.ijrms20150271 Case Report Chediak Higashi Syndrome masquerading as acute leukemia / storage disorder - A rare case report Mirza Asif Baig1,*, Anil Sirasgi2 1Former Asst. professor, BLDUs Shri B.M. Patil Medical College, Bijapure, Karnataka, India 2Associate professor, ESI Medical College, Gulbarga, Karnataka, India Received: 19 April 2015 Revised: 09 May 2015 Accepted: 23 May 2015 *Correspondence: Dr. Mirza Asif Baig, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Chediak higashi Syndrome (CHS) is a rare autosomal recessive multisystem disorder with a defect in granule morphogenesis with giant lysosomes in leucocyte and other cells. CHS is a rare disease, approximately 200 cases have been reported so far. It was described in detail by Chediak in 1952 and Higashi in 1954. 1½ year old male child presented with multiple hypopigment patches on lower extremities, light colored hair, Hepatosplenomegaly and generalised Lymphadenopathy. PBS shows giant prominent liliac to purple granules in neutrophils, band forms, few lymphocytes and monocytes. Bone marrow is hypercellular showing giant prominent gray blue to purple heterogeneous granules often multiple seen in many myeloid precursors, Neutrophils, few lymphocytes and monocytes. -

Clozapine, Agranulocytosis, and Benign Ethnic Neutropenia
EDITORIAL 545 Postgrad Med J: first published as 10.1136/pgmj.2004.031641 on 2 September 2005. Downloaded from Pharmacology and toxicology ethnic groups in the Middle East, ....................................................................................... including Yemenite Jews and Jordanians, have BEN.12 13 BEN has only been reported in ethnic groups that have Clozapine, agranulocytosis, and tanned or dark skin.13 Subjects with BEN do not show increased incidence of benign ethnic neutropenia infections, and their response to infec- tions is similar to those without BEN.13 S Rajagopal ................................................................................... CLINICAL IMPLICATIONS In the United Kingdom and Ireland, the Current knowledge and clinical implications Clozaril patient monitoring service (CPMS) supervises the prescribing of clozapine and the haematological test- lozapine is an atypical antipsycho- agranulocytosis, is more common in ing (Clozaril is the brand name of tic that is effective in treatment black people.6 A white cell count spike clozapine). The CPMS uses a lower cut resistant schizophrenia.1 The of 15% or more above the immediately C off point for patients with BEN than for National Institute for Health and preceding measurement may predict the general population (table 1). A Clinical Excellence (NICE) guidelines agranulocytosis within the next ‘‘green’’ alert indicates satisfactory for schizophrenia specify that ‘‘in indi- 75 days.7 However, as these differences count, an ‘‘amber’’ alert requires a viduals with evidence of treatment between the risk factors for agranulocy- repeat FBC test while clozapine can be resistant schizophrenia, clozapine tosis and neutropenia have been extra- should be introduced at the earliest polated primarily from epidemiological continued, and a ‘‘red’’ alert warrants opportunity’’.2 studies, they may be subject to change immediate cessation of clozapine. -

Severe Agranulocytosis in Two Patients with Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms
Acta Derm Venereol 2016; 96: 842–843 SHORT COMMUNICATION Severe Agranulocytosis in Two Patients with Drug-induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms Miyuki Kato, Yoko Kano*, Yohei Sato and Tetsuo Shiohara Department of Dermatology, Kyorin University School of Medicine, 6-20-2 Shinkawa Mitaka, Tokyo 181-8611, Japan. *E-mail: [email protected] Accepted Mar 24, 2016; Epub ahead of print Mar 30, 2016 Drug-induced hypersensitivity syndrome/drug reac- No evidence was seen of lymphoma or other haematological tion with eosinophilia and systemic symptoms (DIHS/ malignancies. Granulocyte-colony stimulating factor (G-CSF) DRESS) is a life-threatening adverse reaction characteri- and intravenous immunoglobulin at 5 g/day were administered for 5 days. As high-grade fever continued, antibiotics were zed by skin rashes, fever, leukocytosis with eosinophilia started. During the appearance of agranulocytosis, atypical and/or atypical lymphocytosis, lymph node enlargement, lymphocytosis (2–11%) was detected. On day 24 after onset, and liver and/or renal dysfunctions (1, 2). A wide variety leucocyte count was normalized, but liver dysfunction ap- of other involvements have also been reported, including peared (aspartate aminotransferase (AST) 276 IU/l (normal limbic encephalitis, myocarditis, and gastrointestinal < 33 IU/l); alanine aminotransferase (ALT) 159 IU/l (normal < 30 IU/l)). Renal function was also exacerbated (BUN 86.6 disease, developing during the course of the disease mg/dl; Cr 2.8 mg/dl). Seven days later, leucocytes overshot (3–5). It has been demonstrated that human herpesvirus to 21.3 × 109/l (neutrophil 81.0%; eosinophil 0.5%; monocyte 6 (HHV-6), Epstein-Barr virus (EBV) and cytomegalo- 8%; lymphocyte 10%; atypical lymphocyte 0.5%). -

Practice Parameter for the Diagnosis and Management of Primary Immunodeficiency
Practice parameter Practice parameter for the diagnosis and management of primary immunodeficiency Francisco A. Bonilla, MD, PhD, David A. Khan, MD, Zuhair K. Ballas, MD, Javier Chinen, MD, PhD, Michael M. Frank, MD, Joyce T. Hsu, MD, Michael Keller, MD, Lisa J. Kobrynski, MD, Hirsh D. Komarow, MD, Bruce Mazer, MD, Robert P. Nelson, Jr, MD, Jordan S. Orange, MD, PhD, John M. Routes, MD, William T. Shearer, MD, PhD, Ricardo U. Sorensen, MD, James W. Verbsky, MD, PhD, David I. Bernstein, MD, Joann Blessing-Moore, MD, David Lang, MD, Richard A. Nicklas, MD, John Oppenheimer, MD, Jay M. Portnoy, MD, Christopher R. Randolph, MD, Diane Schuller, MD, Sheldon L. Spector, MD, Stephen Tilles, MD, Dana Wallace, MD Chief Editor: Francisco A. Bonilla, MD, PhD Co-Editor: David A. Khan, MD Members of the Joint Task Force on Practice Parameters: David I. Bernstein, MD, Joann Blessing-Moore, MD, David Khan, MD, David Lang, MD, Richard A. Nicklas, MD, John Oppenheimer, MD, Jay M. Portnoy, MD, Christopher R. Randolph, MD, Diane Schuller, MD, Sheldon L. Spector, MD, Stephen Tilles, MD, Dana Wallace, MD Primary Immunodeficiency Workgroup: Chairman: Francisco A. Bonilla, MD, PhD Members: Zuhair K. Ballas, MD, Javier Chinen, MD, PhD, Michael M. Frank, MD, Joyce T. Hsu, MD, Michael Keller, MD, Lisa J. Kobrynski, MD, Hirsh D. Komarow, MD, Bruce Mazer, MD, Robert P. Nelson, Jr, MD, Jordan S. Orange, MD, PhD, John M. Routes, MD, William T. Shearer, MD, PhD, Ricardo U. Sorensen, MD, James W. Verbsky, MD, PhD GlaxoSmithKline, Merck, and Aerocrine; has received payment for lectures from Genentech/ These parameters were developed by the Joint Task Force on Practice Parameters, representing Novartis, GlaxoSmithKline, and Merck; and has received research support from Genentech/ the American Academy of Allergy, Asthma & Immunology; the American College of Novartis and Merck. -

Oral Sequelae of Chronic Neutrophil Defects: Case Report of A
Case Report Oral sequelaeof chronic neutrophil defects: case report of a child with glycogenstorage diseasetype lb Nancy Dougherty, DMDMary Ann Gataletto, DMD complex group of enzymereactions is respon- tion presently are inconclusive. Various etiologies of sible for the breakdownof the large glycogen neutropenia include abnormal maturation of neutro- A moleculeinto glucose, whichis used by the body phil precursors and reduced release of neutrophils from to maintain blood sugar and provide energy. The glyco- the bone marrow.It is unclear whether either of these is gen storage diseases are a group of inherited disorders responsible for the neutropenia seen in GSDtype lb. involving deficiencies of one or more of the enzymes The importance of transport of glucose into neutrophils necessary to store and metabolize glycogen. Glycogen for chemotaxis has been demonstrated, and this might storage disease (GSD)exists in a variety of forms, each well2 be the etiology for the neutrophil dysfunction. involving different enzyme systems of the glycogen The purpose of this paper is to present, via a report metabolic pathway. of a patient with GSDtype lb, the short- and long-term GSDtype lb is caused by a lack of glucose-6-phos- effects of a chronic neutrophil defect on the phatase (G6P) translocase. This prevents the transport periodontium and oral mucosa. of G6Pacross the endoplasmic reticulum.1 As a result, glycogen cannot be metabolized into glucose and is Casereport deposited in the liver. The modeof genetic transmis- Medicalhistory sion of GSDtype lb is autosomal recessive. It is ex- The patient was an African-American male diag- tremely rare, with an estimated incidence of less than 1 nosed with GSDtype lb at 3 months of age.