The Hard Corals (Scleractinia) of India: a Revised Checklist
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

(Symbiodinium) in Scleractinian Corals from Tropical Reefs in Southern Hainan
Journal of Systematics and Evolution 49 (6): 598–605 (2011) doi: 10.1111/j.1759-6831.2011.00161.x Research Article Low genetic diversity of symbiotic dinoflagellates (Symbiodinium) in scleractinian corals from tropical reefs in southern Hainan Island, China 1,2Guo-Wei ZHOU 1,2Hui HUANG∗ 1(Key Laboratory of Marine Bio-resources Sustainable Utilization, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China) 2(Tropical Marine Biological Research Station in Hainan, Chinese Academy of Sciences, Sanya 572000, China) Abstract Endosymbiotic dinoflagellates in the genus Symbiodinium are among the most abundant and important group of photosynthetic protists found in coral reef ecosystems. In order to further characterize this diversity and compare with other regions of the Pacific, samples from 44 species of scleractinian corals representing 20 genera and 9 families, were collected from tropical reefs in southern Hainan Island, China. Denaturing gradient gel electrophoresis fingerprinting of the ribosomal internal transcribed spacer 2 identified 11 genetically distinct Symbiodinium types that have been reported previously. The majority of reef-building coral species (88.6%) harbored only one subcladal type of symbiont, dominated by host-generalist C1 and C3, and was influenced little by the host’s apparent mode of symbiont acquisition. Some species harbored more than one clade of Symbiodinium (clades C, D) concurrently. Although geographically isolated from the rest of the Pacific, the symbiont diversity in southern Hainan Island was relatively low and similar to both the Great Barrier Reef and Hawaii symbiont assemblages (dominated by clade C Symbiodinium). These results indicate that a specialist symbiont is not a prerequisite for existence in remote and isolated areas, but additional work in other geographic regions is necessary to test this idea. -

Taxonomic Checklist of CITES Listed Coral Species Part II
CoP16 Doc. 43.1 (Rev. 1) Annex 5.2 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of CITES listed Coral Species Part II CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families Family Name Accepted Name Species Author Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Madrepora crassa Milne Edwards & Haime, 1860; ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) Madrepora abrotanoides Lamarck, 1816; Acropora mangarevensis Vaughan, 1906 ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Dana, 1846 Madrepora acuminata Verrill, 1864; Madrepora diffusa ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Verrill, 1864; Acropora diffusa (Verrill, 1864); Madrepora nigra Brook, 1892 ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora coronata Brook, 1892; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) anthocercis Brook, 1893 ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Madrepora aspera Dana, 1846; Acropora cribripora (Dana, 1846); Madrepora cribripora Dana, 1846; Acropora manni (Quelch, 1886); Madrepora manni ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Quelch, 1886; Acropora hebes (Dana, 1846); Madrepora hebes Dana, 1846; Acropora yaeyamaensis Eguchi & Shirai, 1977 ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera Dana, 1846 ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, 1997 Veron (2000) ACROPORIDAE Acropora bifurcata Nemenzo, 1971 Veron (2000) ACROPORIDAE Acropora branchi Riegl, 1995 Veron (2000) Madrepora brueggemanni Brook, 1891; Isopora ACROPORIDAE Acropora brueggemanni (Brook, 1891) Veron (2000) brueggemanni (Brook, 1891) ACROPORIDAE Acropora bushyensis Veron & Wallace, 1984 Veron (2000) Acropora fasciculare Latypov, 1992 ACROPORIDAE Acropora cardenae Wells, 1985 Veron (2000) CoP16 Doc. -

Settlement of Larvae from Four Families of Corals in Response to a Crustose Coralline Alga and Its Biochemical Morphogens Taylor N
www.nature.com/scientificreports OPEN Settlement of larvae from four families of corals in response to a crustose coralline alga and its biochemical morphogens Taylor N. Whitman1,2, Andrew P. Negri 1, David G. Bourne 1,2 & Carly J. Randall 1* Healthy benthic substrates that induce coral larvae to settle are necessary for coral recovery. Yet, the biochemical cues required to induce coral settlement have not been identifed for many taxa. Here we tested the ability of the crustose coralline alga (CCA) Porolithon onkodes to induce attachment and metamorphosis, collectively termed settlement, of larvae from 15 ecologically important coral species from the families Acroporidae, Merulinidae, Poritidae, and Diploastreidae. Live CCA fragments, ethanol extracts, and hot aqueous extracts of P. onkodes induced settlement (> 10%) for 11, 7, and 6 coral species, respectively. Live CCA fragments were the most efective inducer, achieving over 50% settlement for nine species. The strongest settlement responses were observed in Acropora spp.; the only non-acroporid species that settled over 50% were Diploastrea heliopora, Goniastrea retiformis, and Dipsastraea pallida. Larval settlement was reduced in treatments with chemical extracts compared with live CCA, although high settlement (> 50%) was reported for six acroporid species in response to ethanol extracts of CCA. All experimental treatments failed (< 10%) to induce settlement in Montipora aequituberculata, Mycedium elephantotus, and Porites cylindrica. Individual species responded heterogeneously to all treatments, suggesting that none of the cues represent a universal settlement inducer. These results challenge the commonly-held notion that CCA ubiquitously induces coral settlement, and emphasize the critical need to assess additional cues to identify natural settlement inducers for a broad range of coral taxa. -

Pleistocene Reefs of the Egyptian Red Sea: Environmental Change and Community Persistence
Pleistocene reefs of the Egyptian Red Sea: environmental change and community persistence Lorraine R. Casazza School of Science and Engineering, Al Akhawayn University, Ifrane, Morocco ABSTRACT The fossil record of Red Sea fringing reefs provides an opportunity to study the history of coral-reef survival and recovery in the context of extreme environmental change. The Middle Pleistocene, the Late Pleistocene, and modern reefs represent three periods of reef growth separated by glacial low stands during which conditions became difficult for symbiotic reef fauna. Coral diversity and paleoenvironments of eight Middle and Late Pleistocene fossil terraces are described and characterized here. Pleistocene reef zones closely resemble reef zones of the modern Red Sea. All but one species identified from Middle and Late Pleistocene outcrops are also found on modern Red Sea reefs despite the possible extinction of most coral over two-thirds of the Red Sea basin during glacial low stands. Refugia in the Gulf of Aqaba and southern Red Sea may have allowed for the persistence of coral communities across glaciation events. Stability of coral communities across these extreme climate events indicates that even small populations of survivors can repopulate large areas given appropriate water conditions and time. Subjects Biodiversity, Biogeography, Ecology, Marine Biology, Paleontology Keywords Coral reefs, Egypt, Climate change, Fossil reefs, Scleractinia, Cenozoic, Western Indian Ocean Submitted 23 September 2016 INTRODUCTION Accepted 2 June 2017 Coral reefs worldwide are threatened by habitat degradation due to coastal development, 28 June 2017 Published pollution run-off from land, destructive fishing practices, and rising ocean temperature Corresponding author and acidification resulting from anthropogenic climate change (Wilkinson, 2008; Lorraine R. -

Final Corals Supplemental Information Report
Supplemental Information Report on Status Review Report And Draft Management Report For 82 Coral Candidate Species November 2012 Southeast and Pacific Islands Regional Offices National Marine Fisheries Service National Oceanic and Atmospheric Administration Department of Commerce Table of Contents INTRODUCTION ............................................................................................................................................. 1 Background ............................................................................................................................................... 1 Methods .................................................................................................................................................... 1 Purpose ..................................................................................................................................................... 2 MISCELLANEOUS COMMENTS RECEIVED ...................................................................................................... 3 SRR EXECUTIVE SUMMARY ........................................................................................................................... 4 1. Introduction ........................................................................................................................................... 4 2. General Background on Corals and Coral Reefs .................................................................................... 4 2.1 Taxonomy & Distribution ............................................................................................................. -

Wallace Et Al MTQ Catalogue Embedded Pics.Vp
Memoirs of the Queensland Museum | Nature 57 Revision and catalogue of worldwide staghorn corals Acropora and Isopora (Scleractinia: Acroporidae) in the Museum of Tropical Queensland Carden C. Wallace, Barbara J. Done & Paul R. Muir © Queensland Museum PO Box 3300, South Brisbane 4101, Australia Phone 06 7 3840 7555 Fax 06 7 3846 1226 Email [email protected] Website www.qm.qld.gov.au National Library of Australia card number ISSN 0079-8835 NOTE Papers published in this volume and in all previous volumes of the Memoirs of the Queensland Museum may be reproduced for scientific research, individual study or other educational purposes. Properly acknowledged quotations may be made but queries regarding the republication of any papers should be addressed to the Director. Copies of the journal can be purchased from the Queensland Museum Shop. A Guide to Authors is displayed at the Queensland Museum web site www.qm.qld.gov.au A Queensland Government Project Typeset at the Queensland Museum Memoirs of the Queensland Museum | Nature Volume 57 Minister: The Honourable Ros Bates, MP, Minister for Science, Information Technology, Innovation and the Arts CEO: I.D. Galloway, PhD Editor in Chief: J.N.A. Hooper, PhD Managing Editor: S.M. Verschoore Issue Editor: P.J.F. Davie PUBLISHED BY ORDER OF THE BOARD 30 JUNE 2012 © The State of Queensland (Queensland Museum), 2012 PO Box 3300, South Brisbane 4101, Qld Australia Phone 61 7 3840 7555 Fax 61 7 3846 1226 www.qm.qld.gov.au National Library of Australia card number ISSN 0079-8835 COVER: Acropora muricata (Linnaeus, 1758), type species of Acropora, Maldives, 2006 (MTQ-G60372), photo: Paul Muir. -

Hermatypic Coral Fauna of Subtropical Southeast Africa: a Checklist!
Pacific Science (1996), vol. 50, no. 4: 404-414 © 1996 by University of Hawai'i Press. All rights reserved Hermatypic Coral Fauna of Subtropical Southeast Africa: A Checklist! 2 BERNHARD RrnGL ABSTRACT: The South African hermatypic coral fauna consists of 96 species in 42 scleractinian genera, one stoloniferous octocoral genus (Tubipora), and one hermatypic hydrocoral genus (Millepora). There are more species in southern Mozambique, with 151 species in 49 scleractinian genera, one stolo niferous octocoral (Tubipora musica L.), and one hydrocoral (Millepora exaesa [Forskal)). The eastern African coral faunas of Somalia, Kenya, Tanzania, Mozambique, and South Africa are compared and Southeast Africa dis tinguished as a biogeographic subregion, with six endemic species. Patterns of attenuation and species composition are described and compared with those on the eastern boundaries of the Indo-Pacific in the Pacific Ocean. KNOWLEDGE OF CORAL BIODIVERSITY in the Mason 1990) or taxonomically inaccurate Indo-Pacific has increased greatly during (Boshoff 1981) lists of the corals of the high the past decade (Sheppard 1987, Rosen 1988, latitude reefs of Southeast Africa. Sheppard and Sheppard 1991 , Wallace and In this paper, a checklist ofthe hermatypic Pandolfi 1991, 1993, Veron 1993), but gaps coral fauna of subtropical Southeast Africa, in the record remain. In particular, tropical which includes the southernmost corals of and subtropical subsaharan Africa, with a Maputaland and northern Natal Province, is rich and diverse coral fauna (Hamilton and evaluated and compared with a checklist of Brakel 1984, Sheppard 1987, Lemmens 1993, the coral faunas of southern Mozambique Carbone et al. 1994) is inadequately docu (Boshoff 1981). -

FDM 2017 Coral Species Reef Survey
Submitted in support of the U.S. Navy’s 2018 Annual Marine Species Monitoring Report for the Pacific Final ® FARALLON DE MEDINILLA 2017 SPECIES LEVEL CORAL REEF SURVEY REPORT Dr. Jessica Carilli, SSC Pacific Mr. Stephen H. Smith, SSC Pacific Mr. Donald E. Marx Jr., SSC Pacific Dr. Leslie Bolick, SSC Pacific Dr. Douglas Fenner, NOAA August 2018 Prepared for U.S. Navy Pacific Fleet Commander Pacific Fleet 250 Makalapa Drive Joint Base Pearl Harbor Hickam Hawaii 96860-3134 Space and Naval Warfare Systems Center Pacific Technical Report number 18-1079 Distribution Statement A: Unlimited Distribution 1 Submitted in support of the U.S. Navy’s 2018 Annual Marine Species Monitoring Report for the Pacific REPORT DOCUMENTATION PAGE Form Approved OMB No. 0704-0188 Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instructions, searching data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to Washington Headquarters Service, Directorate for Information Operations and Reports, 1215 Jefferson Davis Highway, Suite 1204, Arlington, VA 22202-4302, and to the Office of Management and Budget, Paperwork Reduction Project (0704-0188) Washington, DC 20503. PLEASE DO NOT RETURN YOUR FORM TO THE ABOVE ADDRESS. 1. REPORT DATE (DD-MM-YYYY) 2. REPORT TYPE 3. DATES COVERED (From - To) 08-2018 Monitoring report September 2017 - October 2017 4. TITLE AND SUBTITLE 5a. CONTRACT NUMBER FARALLON DE MEDINILLA 2017 SPECIES LEVEL CORAL REEF SURVEY REPORT 5b. -

AC26 Doc. 20 Annex 6 English Only / Únicamente En Inglés / Seulement En Anglais
AC26 Doc. 20 Annex 6 English only / únicamente en inglés / seulement en anglais Annex 6 CORAL SPECIES, SYNONYMS, AND NOMENCLATURE REFERENCES CURRENTLY RECOGNIZED IN THE UNEP-WCMC DATABASE Data kindly provided by UNEP-WCMC AC26 Doc. 20, Annex 6 – 1 AC26 Doc. 20 Annex 6 English only / únicamente en inglés / seulement en anglais CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families FamName Accepted Name SpcAuthor Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Acropora mangarevensis; Madrepora ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) crassa; Madrepora abrotanoides ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Acropora diffusa; Madrepora acuminata; ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Madrepora diffusa; Madrepora nigra ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora anthocercis; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) coronata ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Acropora cribripora; Acropora hebes; Acropora manni; Acropora yaeyamaensis; ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Madrepora aspera; Madrepora cribripora; Madrepora hebes; Madrepora manni ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, -
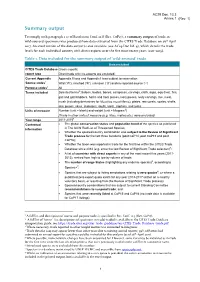
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g. -

Host Traits and Evolution Shape Key Coral-Bacterial Symbioses
Host traits and evolution shape key coral-bacterial symbioses Francesco Ricci ( [email protected] ) University of Melbourne Kshitij Tandon Biodiversity Research Center, Academia Sinica https://orcid.org/0000-0003-3022-0808 Jay Black University of Melbourne Kim-Ahn Lê Cao University of Melbourne Linda Blackall University of Melbourne Heroen Verbruggen University of Melbourne Article Keywords: Tropical Scleractinian Corals, Coral Microbiome, Holobiont, Microbial Reservoir Posted Date: July 29th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-757354/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License 1 Host traits and evolution shape key coral-bacterial symbioses 2 Francesco Riccia, Kshitij Tandona,b, Jay R. Blackc, Kim-Anh Lê Caod, Linda L. Blackalla, 3 Heroen Verbruggena 4 aSchool of BioSciences, University of Melbourne, Victoria, 3010, Australia 5 bBiodiversity Research Center, Academia Sinica, Taipei, 11529, Taiwan 6 cSchool of Geography, Earth and Atmospheric Sciences, University of Melbourne, Victoria, 3010, 7 Australia 8 dMelbourne Integrative Genomics, School of Mathematics and Statistics, University of Melbourne, 9 Victoria, 3010, Australia 10 Abstract 11 The success of tropical scleractinian corals depends on their ability to establish symbioses with 12 microbial partners. Host traits and evolution are known to shape the coral microbiome, but to what 13 extent they affect its composition remains unclear. Here, by using twelve coral species representing the 14 complex and robust clades, we show that functional traits and host evolutionary history explain 14% of 15 the tissue and 13% of the skeletal microbiome composition, providing evidence that these predictors 16 contribute to shaping the holobiont in terms of the presence and abundance of key bacterial species. -

Twenty-Fifth Meeting of the Animals Committee
AC25 Doc. 22 (Rev. 1) Annex 5 (English only / únicamente en inglés / seulement en anglais) Annex 5 Extract of Coral introduction from the ‘Checklist of CITES Species 2008’ (http://www.cites.org/eng/resources/pub/checklist08/index.html). References mentioned in this text can be looked up on the website. CORALS No standard references have been adopted for the coral species listed in the CITES Appendices. Two main references have been used as a basis for the taxonomy of Scleractinia spp., Milleporidae spp. and Stylasteridae spp.: Cairns et al. (1999), supplemented by Veron (2000). Antipatharia spp. have never been the subject of a complete taxonomic revision, although Opresko (1974) provided an incomplete summary. An ongoing revision of the Order by Opresko currently has five parts published (2001-2006), covering the families Aphanipathidae (22 spp.), Cladopathidae (16 spp.), Myriopathidae (32 spp.), Schizopathidae (37 spp.) and Stylopathidae (8 spp.), leaving the Antipathidae (approx. 122 spp.) and the Leiopathidae (6 spp.) to be dealt with. The accepted species of Leiopathidae in the UNEP-WCMC database and most of the accepted species of Antipathidae accord with those accepted by Bisby et al. (2007). A number of species that were not included in the 2005 checklist have been added to the 2008 Checklist. These include species described since 2005; species described before 2005 that were overlooked when producing the 2005 checklist; and species that are now considered to be accepted because of recent taxonomic revisions (Table 1). Others are newly added synonyms (Table 2). A number of other names included in the 2005 checklist have been subsequently modified.