Mississippi-B1
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Protection Against Wave-Based Erosion
Protection against Wavebased Erosion The guidelines below address the elements of shore structure design common to nearly all erosion control structures subject to direct wave action and run-up. 1. Minimize the extent waterward. Erosion control structures should be designed with the smallest waterward footprint possible. This minimizes the occupation of the lake bottom, limits habitat loss and usually results in a lower cost to construct the project. In the case of stone revetments, the crest width should be only as wide as necessary for a stable structure. In general, the revetment should follow the cross-section of the bluff or dune and be located as close to the bluff or dune as possible. For seawalls, the distance that the structure extends waterward of the upland must be minimized. If the seawall height is appropriately designed to prevent the majority of overtopping, there is no engineering rationale based only on erosion control which justifies extending a seawall out into the water. 2. Minimize the impacts to adjacent properties. The design of the structure must consider the potential for damaging adjacent property. Projects designed to extend waterward of the shore will affect the movement of littoral material, reducing the overall beach forming process which in turn may cause accelerated erosion on adjacent or down-drift properties with less protective beaches. Seawalls, (and to a lesser extent, stone revetments) change the direction (wave reflection) and intensity of wave energy along the shore. Wave reflection can cause an increase in the total energy at the seawall or revetment interface with the water, allowing sand and gravel to remain suspended in the water, which will usually prevent formation of a beach directly fronting the structure. -

Marine Nearshore Restoration Recommendations Whatcom County Shoreline Management Project
Marine Nearshore Restoration Recommendations Whatcom County Shoreline Management Project 1 7 Old Fish 6 Packers Pier Tongue Point Blaine Marina Site Specific Recommendations t pi S o o hm ia m e S Semiahmoo Restoration Site 5 Marina Shoreline Reach Breaks The large platform and foundation could be removed to restore the beach and fringing marsh D Shoreline Modifications 1 2 a kota Cr 3 Removal of bulkheads that protrude into 4 Retaining Walls Remove the intertidal dilapidated Groins and Jetties dock 5 6 Miscellaneous Structures 1 7 C al 8 Piers ifo rn ia 9 2 C r Platforms 4 3 C r 4 nd Bulkheads ra rt Birch Point 5 e Outfall PipesB Cottonwood Beach 6 Building (Shorelines Only) 7 Administrative Boundries r 2 e Birch Bay v Village Marina 3 i 8 R Lummi Nation k Remove groins and bulkheads c a along Birch Bay Drive to restore upper s k beach and backshore habitats Whatcom County oo N e m ns t Mai 2 For more information on restoration sites, includi1ng non site-specific recommendations, see the Whatcom County Shoreline Management Project Inventory & 1 Characterization Report (Backgr1ound document Vol. I) and the Marine Resources Committee Document, Restoration Recommendations by Shoreline Reach by Coastal Ge1o1logic Services and Adolfson and9 Assoc1ia0 tes (2006). 2 DATA SOURCES: Restoration Sites - Coastal Geologic Remove bulkheads along these bluffs, which are the sole Services, Inc., Mod8ifications - WC 2005 (Pictometry 2004), T sediment source for accretionary shoreforms and valuable er r ell C Outfall Pipes - REsources, DNR, Pictometry, Contour lines 2 habitat in Birch Bay and State Park reaches r 1 10 meter intervals, USGS Elevation labels in feet. -

Aquatic Fish Report
Aquatic Fish Report Acipenser fulvescens Lake St urgeon Class: Actinopterygii Order: Acipenseriformes Family: Acipenseridae Priority Score: 27 out of 100 Population Trend: Unknown Gobal Rank: G3G4 — Vulnerable (uncertain rank) State Rank: S2 — Imperiled in Arkansas Distribution Occurrence Records Ecoregions where the species occurs: Ozark Highlands Boston Mountains Ouachita Mountains Arkansas Valley South Central Plains Mississippi Alluvial Plain Mississippi Valley Loess Plains Acipenser fulvescens Lake Sturgeon 362 Aquatic Fish Report Ecobasins Mississippi River Alluvial Plain - Arkansas River Mississippi River Alluvial Plain - St. Francis River Mississippi River Alluvial Plain - White River Mississippi River Alluvial Plain (Lake Chicot) - Mississippi River Habitats Weight Natural Littoral: - Large Suitable Natural Pool: - Medium - Large Optimal Natural Shoal: - Medium - Large Obligate Problems Faced Threat: Biological alteration Source: Commercial harvest Threat: Biological alteration Source: Exotic species Threat: Biological alteration Source: Incidental take Threat: Habitat destruction Source: Channel alteration Threat: Hydrological alteration Source: Dam Data Gaps/Research Needs Continue to track incidental catches. Conservation Actions Importance Category Restore fish passage in dammed rivers. High Habitat Restoration/Improvement Restrict commercial harvest (Mississippi River High Population Management closed to harvest). Monitoring Strategies Monitor population distribution and abundance in large river faunal surveys in cooperation -

LOUISIANA K. Meyer-Arendt Department of Geography
65. USA--LOUISIANA K. Meyer-Arendt D.W. Davis Department of Geography Department of Earth Science Mississippi State University Nicholls State University Starkville, Mississippi 38759 Thibodaux, Louisiana 70301 United States of America United States of America INTRODUCTION Louisiana's 40,000 Inn 2 coastal zone developed over the last 7,000 years by the progradation, aggradation, and accretion of sediments introduced via various courses of the Mississippi River (Frazier 1967). The deltaic plain (32,000 km'), through which the modern river cuts diagon ally !Fig , 1), consists of vast wetlands and waterbodies. With eleva tions ranging from sea level up to 1.5 m, it is interrupted by natural levee ridges which decrease distally until they disappear beneath the marsh surface. The downdrift chenier plain of southwest Louisiana (8,000 km') consists of marshes, large round-to-oblong lakes, and stranded, oak covered beach ridges known as cheniers (Howe et al. 1935). This landscape is the result of alternating long-term phases of shoreline accretion and erosion that were dependent upon the proximit of an active sediment-laden river, and a low-energy marine environment (Byrne et al. 1959). Since the dyking of the Mississippi River, fluvial sedimentation in the deltaic plain has effectively been halted. Today, most Missis sippi River sediment is deposited on the outer continental shelf; only at the mouth of the Atchafalaya River distributary is deltaic sedimen tation subaerially significant (Adams and Baumann 1980). Over mos of the coastal zone, subsidence, saltwater intrusion, wave erosion, canalization, and other hydrologic modification have led to a rapid increase in the surface area of water (Davis 1986, Walker e al. -

MF2294 Streambank Revetment
Outdated Publication, for historical use. CAUTION: Recommendations in this publication may be obsolete. Kansas State University Agricultural Experiment Station and Cooperative Extension Service Kansas State University, Division of Biology Kansas Cooperative Fish and Wildlife Research Unit U.S. Environmental Protection Agency Kansas Department of Wildlife & Parks STREAMBANK REVETMENT 1 Outdated Publication, for historical use. CAUTION: Recommendations in this publication may be obsolete. Introduction Streambank erosion is a naturally occurring process in streams and rivers throughout the United States. Accelerated streambank erosion occurs when natural events or human activities cause a higher than expected amount of erosion, and is typically a result of reduced or eliminated riparian (streamside) vegetation. The removal of riparian vegetation is the primary factor influenc- ing streambank stability. Historically, channel straightening (channelization) was the primary method used to control streambank erosion. However, since the 1970s, riparian and in-stream habitat restoration by natural or artificial meth- ods has grown in popularity because channel- ization typically caused problems, such as ero- sion and flooding dowstream. Natural resource agencies throughout the Midwest have been using tree revetments as one type of streambank stabilization structure. What are tree revetments? Tree revetments are a series of trees laid in the stream along the eroding bank. They are de- signed to reduce water velocity, increase siltation within the trees, and reduce slumping of the streambank. Tree revetments are not designed to permanently stabilize eroding streambanks. They should stabilize the streambank until other stabi- lization techniques, such as tree plantings in the riparian area become established. Tree revet- ments are not designed to fix problems at a watershed level. -

Channel Stabilization Publications Available in Corps of Engineers Offices
TECHNICAL REPORT NO. 4 CHANNEL STABILIZATION PUBLICATIONS AVAILABLE IN CORPS OF ENGINEERS OFFICES i <SESS> ¡01 101 LfU U-U lOi 00¡DE November 1966 Committee on Channel Stabilization CORPS OF ENGINEERS, U. S. ARMY REPORTS OF COMMITTEE ON CHANNEL STABILIZATION ¿i BUREAU OF RECLAMATION DENVER Lll 92035635 VT ■ieD3Sb3S nsJjfe-» TECHNICAL REPORT 4 7 3 CHANNEL STABILIZATION PUBLICATIONS AVAILABLE IN CORPS OF ENGINEERS OFFICES j f November 1966 f Committee on Channel Stabilization - i / y > CORPS OF ENGINEERS/ U. S. ARMY ARM Y-MRC VICKSBURG. MISS. PRESENT MEMBERSHIP OF COMMITTEE ON CHANNEL STABILIZATION J. H. Douma Office, Chief of Engineers Chairman E. B. Lipscomb Lower Mississippi Valley Division Recorder D. C. Bondurant Missouri River Division R. H. Haas Lower Mississippi Valley Division W. E. Isaacs Little Rock District C. P. Lindner South Atlantic Division E. B. Madden Southwestern Division H. A. Smith North Pacific Division J. B. Tiffany Waterways Experiment Station G. B. Fenwick Consultant FOREWORD Establishment of the Committee on Channel Stabilization in April 1962 was confirmed by Engineer Regulation 15-2-1, dated 1 November 1962. As stated in ER 15-2-1, the objectives of the Committee with respect to channel stabilization are: a. To review and evaluate pertinent information and disseminate the results thereof. b. To determine the need for and recommend a program of research; and to have advisory technical review responsibility for research assigned to the Committee. £. To determine basic principles and design criteria. d. To provide, at the request of field offices, advice on design and operational problems. In accordance with the desire of the Committee to inventory available data, reports, papers, etc., pertaining to channel stabilization, arrangements were made for the Research Center Library, U. -

Tree Revetments
Tree Revetments Tree revetments are cut increase bank erosion. It trees anchored at the bottom is extremely important (or toe) of unstable stream- to anchor each tree in the banks. These anchored trees revetment at the base or toe serve to slow the current of the eroding bank. This is along the bank, decreasing the point of the bank where erosion and allowing the vertical bank meets the sediment to be deposited horizontal bottom (Figure 2). within the tree branches. If the trees are anchored too Trees with many fine limbs high on the bank, the water and branches are best at may undercut the structure. slowing near-bank currents, If they are placed out in the catching sediment carried channel too far, the current in the stream, and catching Figure 1. Cedar revetment and willow stakes after 3 months. will continue to erode the slump material from the bank. bank behind the revetment. example of this would be stream For this reason, eastern redcedar Another consideration is the soil straightening (or channelization). is usually the best choice. Eastern type and texture that the anchors One indication of this situation redcedar is also more resistant to will be driven into. Sandy or rocky is when a streambank is covered decay than hardwood trees. soils will usually require a larger with trees and vegetation and is The sediment trapped in and anchor driven to a greater depth still eroding. If that is the case, behind revetments provides a while smaller anchors may be a tree revetment may not work. moist, fertile seedbed for vegetation used in heavier clay soils. -

Louisiana's Animal Species of Greatest Conservation Need (SGCN)
Louisiana's Animal Species of Greatest Conservation Need (SGCN) ‐ Rare, Threatened, and Endangered Animals ‐ 2020 MOLLUSKS Common Name Scientific Name G‐Rank S‐Rank Federal Status State Status Mucket Actinonaias ligamentina G5 S1 Rayed Creekshell Anodontoides radiatus G3 S2 Western Fanshell Cyprogenia aberti G2G3Q SH Butterfly Ellipsaria lineolata G4G5 S1 Elephant‐ear Elliptio crassidens G5 S3 Spike Elliptio dilatata G5 S2S3 Texas Pigtoe Fusconaia askewi G2G3 S3 Ebonyshell Fusconaia ebena G4G5 S3 Round Pearlshell Glebula rotundata G4G5 S4 Pink Mucket Lampsilis abrupta G2 S1 Endangered Endangered Plain Pocketbook Lampsilis cardium G5 S1 Southern Pocketbook Lampsilis ornata G5 S3 Sandbank Pocketbook Lampsilis satura G2 S2 Fatmucket Lampsilis siliquoidea G5 S2 White Heelsplitter Lasmigona complanata G5 S1 Black Sandshell Ligumia recta G4G5 S1 Louisiana Pearlshell Margaritifera hembeli G1 S1 Threatened Threatened Southern Hickorynut Obovaria jacksoniana G2 S1S2 Hickorynut Obovaria olivaria G4 S1 Alabama Hickorynut Obovaria unicolor G3 S1 Mississippi Pigtoe Pleurobema beadleianum G3 S2 Louisiana Pigtoe Pleurobema riddellii G1G2 S1S2 Pyramid Pigtoe Pleurobema rubrum G2G3 S2 Texas Heelsplitter Potamilus amphichaenus G1G2 SH Fat Pocketbook Potamilus capax G2 S1 Endangered Endangered Inflated Heelsplitter Potamilus inflatus G1G2Q S1 Threatened Threatened Ouachita Kidneyshell Ptychobranchus occidentalis G3G4 S1 Rabbitsfoot Quadrula cylindrica G3G4 S1 Threatened Threatened Monkeyface Quadrula metanevra G4 S1 Southern Creekmussel Strophitus subvexus -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

Factors Influencing Community Structure of Riverine
FACTORS INFLUENCING COMMUNITY STRUCTURE OF RIVERINE ORGANISMS: IMPLICATIONS FOR IMPERILED SPECIES MANAGEMENT by David S. Ruppel, M.S. A dissertation submitted to the Graduate Council of Texas State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy with a Major in Aquatic Resources and Integrative Biology May 2019 Committee Members: Timothy H. Bonner, Chair Noland H. Martin Joseph A. Veech Kenneth G. Ostrand James A. Stoeckel COPYRIGHT by David S. Ruppel 2019 FAIR USE AND AUTHOR’S PERMISSION STATEMENT Fair Use This work is protected by the Copyright Laws of the United States (Public Law 94-553, section 107). Consistent with fair use as defined in the Copyright Laws, brief quotations from this material are allowed with proper acknowledgement. Use of this material for financial gain without the author’s express written permission is not allowed. Duplication Permission As the copyright holder of this work I, David S. Ruppel, authorize duplication of this work, in whole or in part, for educational or scholarly purposes only. ACKNOWLEDGEMENTS First, I thank my major advisor, Timothy H. Bonner, who has been a great mentor throughout my time at Texas State University. He has passed along his vast knowledge and has provided exceptional professional guidance and support with will benefit me immensely as I continue to pursue an academic career. I also thank my committee members Dr. Noland H. Martin, Dr. Joseph A. Veech, Dr. Kenneth G. Ostrand, and Dr. James A. Stoeckel who provided great comments on my dissertation and have helped in shaping manuscripts that will be produced in the future from each one of my chapters. -
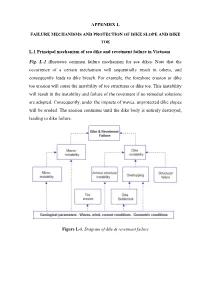
APPENDIX L L.1 Principal Mechanism of Sea Dike and Revetment Failure In
APPENDIX L FAILURE MECHANISMS AND PROTECTION OF DIKE SLOPE AND DIKE TOE L.1 Principal mechanism of sea dike and revetment failure in Vietnam Fig. L-1 illustrates common failure mechanism for sea dikes. Note that the occurrence of a certain mechanism will sequentially result in others, and consequently leads to dike breach. For example, the foreshore erosion or dike toe erosion will cause the instability of toe structures or dike toe. This instability will result in the instability and failure of the revetment if no remedial solutions are adopted. Consequently, under the impacts of waves, unprotected dike slopes will be eroded. The erosion continues until the dike body is entirely destroyed, leading to dike failure. Figure L-1. Diagram of dike & revetment failure 4 2 3 DWL 0 i 1 h original cross-shore profile h at design situation scour holes 1- instability of toe structures 2- instability of slope protection 3- erosion of outer slope 4- erosion of dike crest and inner slope Foreshore erosion (3) Instability of toe protection structures (1) Overflow due to foreshore erosion and local erosion of dike toe (1a) Overtopping; resulting in erosion (4) Inner slope sliding of crest and inner slope (1b) Overtopping; resulting in inner (5) Outer slope sliding slope sliding (6) Internal erosion; piping (2) Instability of outer amour structures/dike slope and body erosion Figure L-2. Typical failure of sea dike In the following sections, details of common failure mechanism of sea dike and revetment system in Vietnam will be given. L.1.1 Wave overtopping Wave overtopping is the dominant mechanism of sea dike failure in Vietnam, as most of sea dikes are overtopped during storms and flood, even in the long-lasting monsoon period. -

A Checklist of Texas Fresh-Water Fishes By
A Checklist of I Texas Fresh-Water Fishes By CLARK HUBBS Department of Zoology The University of Texas DIVISION OF INLAND FISHERIES TEXAS GAME AND FISH COMMISSION Austin, Texas Marion Toole, Director IF Series - No. 3 Revised Dec. 1958 FOREWORD A checklist of Texas fresh-water fishes by CLARK HUBBS This checklist is modified from that of Hubbs (1957a). A number of changes have been made in nomenclature. Notropis roseus and N. deliciosus have been changed to N. texanus and N. strarnineus, re spectively, following Suttkus (1958). Etheostorna whipplei and E. m·tesiae have been changed to E. radiosum following a re-examina tion of available material. Two species have been added, Garnbusia senilis, following Hubbs (1958), and Eucinoslornus argenteus, a marine species collected in a .coastal stream near Brownsville. Two species which had not been described in the previous checklist are given their names, Gambusia geiseri, following Hubbs and Springer (1957), and G. helerochir, following Hubbs (1957b). The primary difference between the checklists is the addition of information on the distribution of fishes within the state to this list. The general concepts follow those given in a previous report (Hubbs, 1957c), but emphasize the ranges of the individual species rather than the distributional patterns. The range designations follow the common names of all species. The numbers refer to the modified game areas shovvn on the map. If the fish inhabits only a part of the area, it is so designated by preceding the number with letters (N. for north, etc.) indicating· the pai·t of the area inhabited by the species.