Approach to Aspiration and Injections ACR Virtual Rheumatology Practicum
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Recurrent Knee Effusions in Gymnast
12-648 LC WHITE Mid Atlantic Regional Chapter of the American College of Sports Medicine Annual Scientific Meeting, November 2nd - 3rd, 2018 Conference Proceedings International Journal of Exercise Science, Issue 9, Volume 7 Recurrent Knee Effusions in Gymnast Stephanie A. Carey, Penn State Milton S. Hershey Medical Center, Hershey, PA. email: [email protected] (Sponsor: Shawn Phillips, MD) History: A 20-year-old current college freshman sustained a right knee effusion following a hyperextension injury approximately 8 years ago while participating in gymnastics. Per report, workup at the time was negative, and she returned to gymnastics. She participated in gymnastics for 2 additional years and retired due to other interests. While continuing regular exercise, and participation in marching band, she reports recurrent, intermittent right knee effusions since that time. She reports that these would occur more often with repetitive activity. Over the past few months, her knee has been more significantly and persistently swollen. She exercises often, but reports no specific inciting incident. She reports pain with end range flexion. She denies any instability or locking. Previous physical therapy has improved her pain. Physical Examination: Examination revealed significant effusion of right knee. No obvious effusions in other joints. Range of motion was normal and pain free. Negative Lachman, anterior drawer, posterior drawer, varus and valgus stress testing , patellar grind, McMurray, Thessaly. Neurovascularly intact. Differential Diagnosis: 1. Meniscal tear, 2 Infection including possible Lyme Disease or Gonococcal Infection; 3. Rheumatoid Arthritis; 4. Gout; 5. Pigmented Villonodular Synovitis; 6. Hemophilia Test and Results: Aspiration: Bloody - >10000 RBCs, no crystals, normal WBC. -

Synovial Joints Permit Movements of the Skeleton
8 Joints Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Joint Structure and Movement Learning Outcomes 8.1 Contrast the major categories of joints, and explain the relationship between structure and function for each category. 8.2 Describe the basic structure of a synovial joint, and describe common accessory structures and their functions. 8.3 Describe how the anatomical and functional properties of synovial joints permit movements of the skeleton. © 2018 Pearson Education, Inc. Section 1: Joint Structure and Movement Learning Outcomes (continued) 8.4 Describe flexion/extension, abduction/ adduction, and circumduction movements of the skeleton. 8.5 Describe rotational and special movements of the skeleton. © 2018 Pearson Education, Inc. Module 8.1: Joints are classified according to structure and movement Joints, or articulations . Locations where two or more bones meet . Only points at which movements of bones can occur • Joints allow mobility while preserving bone strength • Amount of movement allowed is determined by anatomical structure . Categorized • Functionally by amount of motion allowed, or range of motion (ROM) • Structurally by anatomical organization © 2018 Pearson Education, Inc. Module 8.1: Joint classification Functional classification of joints . Synarthrosis (syn-, together + arthrosis, joint) • No movement allowed • Extremely strong . Amphiarthrosis (amphi-, on both sides) • Little movement allowed (more than synarthrosis) • Much stronger than diarthrosis • Articulating bones connected by collagen fibers or cartilage . Diarthrosis (dia-, through) • Freely movable © 2018 Pearson Education, Inc. Module 8.1: Joint classification Structural classification of joints . Fibrous • Suture (sutura, a sewing together) – Synarthrotic joint connected by dense fibrous connective tissue – Located between bones of the skull • Gomphosis (gomphos, bolt) – Synarthrotic joint binding teeth to bony sockets in maxillae and mandible © 2018 Pearson Education, Inc. -

Effectiveness of Distal Tibial Osteotomy
Nozaka et al. BMC Musculoskeletal Disorders (2020) 21:31 https://doi.org/10.1186/s12891-020-3061-7 RESEARCH ARTICLE Open Access Effectiveness of distal tibial osteotomy with distraction arthroplasty in varus ankle osteoarthritis Koji Nozaka* , Naohisa Miyakoshi, Takeshi Kashiwagura, Yuji Kasukawa, Hidetomo Saito, Hiroaki Kijima, Shuichi Chida, Hiroyuki Tsuchie and Yoichi Shimada Abstract Background: In highly active older individuals, end-stage ankle osteoarthritis has traditionally been treated using tibiotalar arthrodesis, which provides considerable pain relief. However, there is a loss of ankle joint movement and a risk of future arthrosis in the adjacent joints. Distraction arthroplasty is a simple method that allows joint cartilage repair; however, the results are currently mixed, with some reports showing improved pain scores and others showing no improvement. Distal tibial osteotomy (DTO) without fibular osteotomy is a type of joint preservation surgery that has garnered attention in recent years. However, to our knowledge, there are no reports on DTO with joint distraction using a circular external fixator. Therefore, the purpose of this study was to examine the effect of DTO with joint distraction using a circular external fixator for treating ankle osteoarthritis. Methods: A total of 21 patients with medial ankle arthritis were examined. Arthroscopic synovectomy and a microfracture procedure were performed, followed by angled osteotomy and correction of the distal tibia; the ankle joint was then stabilized after its condition improved. An external fixator was used in all patients, and joint distraction of approximately 5.8 mm was performed. All patients were allowed full weight-bearing walking immediately after surgery. Results: The anteroposterior and lateral mortise angle during weight-bearing, talar tilt angle, and anterior translation of the talus on ankle stress radiography were improved significantly (P < 0.05). -

HYALURONIC ACID in KNEE OSTEOARTHRITIS Job Hermans
HYALURONIC ACID IN KNEE OSTEOARTHRITIS IN KNEE OSTEOARTHRITIS ACID HYALURONIC HYALURONIC ACID IN KNEE OSTEOARTHRITIS effectiveness and efficiency Job Hermans Job Hermans Hyaluronic Acid in Knee Osteoarthritis effectiveness and efficiency Job Hermans Part of the research described in this thesis was supported by a grant from ZonMW. Financial support for the publication of this thesis was kindly provided by: • Erasmus MC Department of Orthopaedics and Sports Medicine • Nederlandse Orthopaedische Vereniging • Anna Fonds | NOREF • Apotheekgroep Breda • Össur Eindhoven • Bioventus The e-book version of this thesis is available at www.orthopeden.org/downloads/proefschriften ISBN 978-94-6416-168-7 Coverdesign and layout: Publiss.nl Printing: Ridderprint | www.ridderprint.nl © Job Hermans 2020 All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any other information storage or retrieval system, without the prior written permission of the holder of the copyright. Hyaluronic Acid in Knee Osteoarthritis effectiveness and efficiency Hyaluronzuur bij Knieartrose effectiviteit en efficiëntie Thesis to obtain the degree of Doctor from the Erasmus University Rotterdam by command of the rector magnificus Prof.dr. R.C.M.E. Engels and in accordance with the decision of the Doctorate Board. The public defense shall be held on November 24 2020 at 13:30hrs by Job Hermans Born in Boxmeer, the Netherlands Doctoral Committee Promotors Prof.dr. S.M.A. Bierma-Zeinstra Prof.dr. J.A.N. Verhaar Other members Prof.dr. S.K. Bulstra Prof.dr. J.M.W. Hazes Prof.dr. B.W. -

Synovial Fluidfluid 11
LWBK461-c11_p253-262.qxd 11/18/09 6:04 PM Page 253 Aptara Inc CHAPTER SynovialSynovial FluidFluid 11 Key Terms ANTINUCLEAR ANTIBODY ARTHROCENTESIS BULGE TEST CRYSTAL-INDUCED ARTHRITIS GROUND PEPPER HYALURONATE MUCIN OCHRONOTIC SHARDS RHEUMATOID ARTHRITIS (RA) RHEUMATOID FACTOR (RF) RICE BODIES ROPE’S TEST SEPTIC ARTHRITIS Learning Objectives SYNOVIAL SYSTEMIC LUPUS ERYTHEMATOSUS 1. Define synovial. VISCOSITY 2. Describe the formation and function of synovial fluid. 3. Explain the collection and handling of synovial fluid. 4. Describe the appearance of normal and abnormal synovial fluids. 5. Correlate the appearance of synovial fluid with possible cause. 6. Interpret laboratory tests on synovial fluid. 7. Suggest further testing for synovial fluid, based on preliminary results. 8. List the four classes or categories of joint disease. 9. Correlate synovial fluid analyses with their representative disease classification. 253 LWBK461-c11_p253-262.qxd 11/18/09 6:04 PM Page 254 Aptara Inc 254 Graff’s Textbook of Routine Urinalysis and Body Fluids oint fluid is called synovial fluid because of its resem- blance to egg white. It is a viscous, mucinous substance Jthat lubricates most joints. Analysis of synovial fluid is important in the diagnosis of joint disease. Aspiration of joint fluid is indicated for any patient with a joint effusion or inflamed joints. Aspiration of asymptomatic joints is beneficial for patients with gout and pseudogout as these fluids may still contain crystals.1 Evaluation of physical, chemical, and microscopic characteristics of synovial fluid comprise routine analysis. This chapter includes an overview of the composition and function of synovial fluid, and laboratory procedures and their interpretations. -

Adult and Adolescent Knee Pain Guideline Overview
Adult and Adolescent Knee Pain Guideline Overview This Guideline was adapted from and used with the permission of The UW Medical Foundation, UW Hospitals and Clinics, Meriter Hospital, University of Wisconsin Department of Family Medicine, Unity Health Insurance, Physicians Plus Insurance Corporation, and Group Health Cooperative, who created this guideline on May 18, 2007 as the result of a multidisciplinary work group comprised of health care practitioners from orthopedics, sports medicine, and rheumatology. This Guideline was reviewed and approved by Aspirus Network’s Medical Management Committee on May 7, 2013. The Knee Pain Work Group, a multidisciplinary work group comprised of health care practitioners from family practice, internal medicine, pediatric, and orthopedic surgery, participated in the development of this guideline. This guideline is intended to assist the patient-provider team to achieve the “Triple Aim”: quality, cost-efficient care with improved patient experiences / outcomes (i.e. do what’s best for the patient). Any distribution outside of Aspirus Network, Inc. is prohibited. Page 1 of 6 Adult and Adolescent Knee Pain Guideline Overview Guidelines are designed to assist clinicians by providing a framework for the evaluation and treatment of patients. This guideline outlines the preferred approach for most patients. It is not intended to replace a clinician’s judgment or to establish a protocol for all patients. It is understood that some patients will not fit the clinical condition contemplated by a guideline and that a guideline will rarely establish the only appropriate approach to a problem. TABLE OF CONTENTS 1. Patient Presents with Knee Pain ...................................................................... 3 2. History and Physical Exam ............................................................................. -

Pseudogout at the Knee Joint Will Frequently Occur After Hip Fracture
Harato and Yoshida Journal of Orthopaedic Surgery and Research (2015) 10:4 DOI 10.1186/s13018-014-0145-9 RESEARCH ARTICLE Open Access Pseudogout at the knee joint will frequently occur after hip fracture and lead to the knee pain in the early postoperative period Kengo Harato1,3*† and Hiroki Yoshida2† Abstract Background: Symptomatic knee joint effusion is frequently observed after hip fracture, which may lead to postoperative knee pain during rehabilitation after hip fracture surgery. However, unfortunately, very little has been reported on this phenomenon in the literature. The purpose of the current study was to investigate the relationship between symptomatic knee effusion and postoperative knee pain and to clarify the reason of the effusion accompanied by hip fracture. Methods: A total of 100 patients over 65 years of age with an acute hip fracture after fall were prospectively followed up. Knee effusion was assessed on admission and at the operating room before the surgery. If knee effusion was observed at thetimeofthesurgery,synovialfluidwascollectedintosyringes to investigate the cause of the effusion using a compensated polarized light microscope. Furthermore, for each patient, we evaluated age, sex, radiographic knee osteoarthritis (OA), type of the fracture, laterality, severity of the fracture, and postoperative knee pain during rehabilitation. These factors were compared between patients with and without knee effusion at the time of the surgery. As a statistical analysis, we used Mann–Whitney U-test for patients’ age and categorical variables were analyzed by chi-square test or Fisher’sexacttest. Results: A total of 30 patients presented symptomatic knee effusion at the time of the surgery. -

ICD~10~PCS Complete Code Set Procedural Coding System Sample
ICD~10~PCS Complete Code Set Procedural Coding System Sample Table.of.Contents Preface....................................................................................00 Mouth and Throat ............................................................................. 00 Introducton...........................................................................00 Gastrointestinal System .................................................................. 00 Hepatobiliary System and Pancreas ........................................... 00 What is ICD-10-PCS? ........................................................................ 00 Endocrine System ............................................................................. 00 ICD-10-PCS Code Structure ........................................................... 00 Skin and Breast .................................................................................. 00 ICD-10-PCS Design ........................................................................... 00 Subcutaneous Tissue and Fascia ................................................. 00 ICD-10-PCS Additional Characteristics ...................................... 00 Muscles ................................................................................................. 00 ICD-10-PCS Applications ................................................................ 00 Tendons ................................................................................................ 00 Understandng.Root.Operatons..........................................00 -
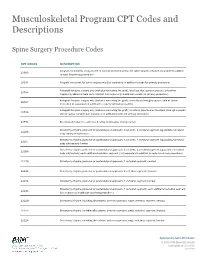
Musculoskeletal Program CPT Codes and Descriptions
Musculoskeletal Program CPT Codes and Descriptions Spine Surgery Procedure Codes CPT CODES DESCRIPTION Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition 20930 to code for primary procedure) 20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar 20936 fragments) obtained from same incision (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial 20937 incision) (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate 20938 skin or fascial incision) (List separately in addition to code for primary procedure) 20974 Electrical stimulation to aid bone healing; noninvasive (nonoperative) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22206 body subtraction); thoracic Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22207 body subtraction); lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22208 body subtraction); each additional vertebral segment (List separately in addition to code for -

CPT® Procedural Coding 110 L with Areportoftheprocedure
20610-20611 2017 Illustrated Coding and Billing Expert for Orthopedics Lower 20610-20611 ICD-9-CM Diagnostic Codes M16.7 Other unilateral secondary 711.05 Pyogenic arthritis involving pelvic osteoarthritis of hip 20610 Arthrocentesis, aspiration and/or region and thigh M17.0 Bilateral primary osteoarthritis of injection, major joint or bursa (eg, 711.06 Pyogenic arthritis involving lower leg knee shoulder, hip, knee, subacromial 713.5 Arthropathy associated with ⇄ M17.11 Unilateral primary osteoarthritis, right bursa); without ultrasound guidance neurological disorders knee 20611 Arthrocentesis, aspiration and/or 714.0 Rheumatoid arthritis ⇄ M17.12 Unilateral primary osteoarthritis, left knee injection, major joint or bursa (eg, 715.15 Osteoarthrosis, localized, primary, pelvic region and thigh M17.2 Bilateral post-traumatic osteoarthritis shoulder, hip, knee, subacromial 715.16 Osteoarthrosis, localized, primary, of knee bursa); with ultrasound guidance, with lower leg M17.5 Other unilateral secondary permanent recording and reporting 715.25 Osteoarthrosis, localized, secondary, osteoarthritis of knee (Do not report 20610, 20611 in pelvic region and thigh ⇄ M1A.051 Idiopathic chronic gout, right hip conjunction with 27370, 76942) 715.26 Osteoarthrosis, localized, secondary, ⇄ M1A.062 Idiopathic chronic gout, left knee (If fluoroscopic, CT, or MRI guidance is lower leg ⇄ M25.052 Hemarthrosis, left hip ⇄ M25.061 Hemarthrosis, right knee performed, see 77002, 77012, 77021) 715.35 Osteoarthrosis, localized, not specified whether primary -

Prolotherapy: a Nontraditional Approach to Knee Osteoarthritis
® Priority updates from the research literature PURLs from the family Physicians inquiries network Andrew H. Slattengren, DO; Trent Christensen, MD; Shailendra Prasad, Prolotherapy: A nontraditional MBBS, MPH; Kohar Jones, MD North Memorial Family approach to knee osteoarthritis Medicine Residency, University of Minnesota, Minneapolis (Drs. Dextrose injections into the knee can reduce pain and Slattengren, Christensen, and Prasad); Department improve a patient’s quality of life. of Family Medicine, The University of Chicago (Dr. Jones) PURL s E D i tor Kate Rowland, MD, MS Department of Family PRACTICE CHANGER acid, and corticosteroid injections. Cost, ef- Medicine, The University Recommend prolotherapy for patients with ficacy, and safety limit these therapies.3 of Chicago knee osteoarthritis (OA) that does not re- Prolotherapy is another option used spond to conventional therapies.1 to treat musculoskeletal pain. It involves repeatedly injecting a sclerosing solution STRENGTH OF RECOMMENDATiON (usually dextrose) into the sites of chronic B: Based on a 3-arm, blinded, randomized musculoskeletal pain.4 The mechanism of controlled trial (RCT). action is thought to be the result of local tis- Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for sue irritation stimulating inflammatory path- knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237. ways, which leads to the release of growth factors and subsequent healing.4,5 Previous studies evaluating the usefulness of prolo- ILLUSTRATIVE CASE therapy have lacked methodological rigor, a 59-year-old woman with OA comes to your have not been randomized adequately, or office with chronic knee pain. She has tried ac- have lacked a placebo comparison.6-9 etaminophen, ibuprofen, intra-articular cortico- steroid injections, and physical therapy without significant improvement in pain or functioning. -

Priority Health Spine and Joint Code List
Priority Health Joint Services Code List Category CPT® Code CPT® Code Description Joint Services 23000 Removal of subdeltoid calcareous deposits, open Joint Services 23020 Capsular contracture release (eg, Sever type procedure) Joint Services 23120 Claviculectomy; partial Joint Services 23130 Acromioplasty or acromionectomy, partial, with or without coracoacromial ligament release Joint Services 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Joint Services 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open;chronic Joint Services 23415 Coracoacromial ligament release, with or without acromioplasty Joint Services 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Joint Services 23430 Tenodesis of long tendon of biceps Joint Services 23440 Resection or transplantation of long tendon of biceps Joint Services 23450 Capsulorrhaphy, anterior; Putti-Platt procedure or Magnuson type operation Joint Services 23455 Capsulorrhaphy, anterior;with labral repair (eg, Bankart procedure) Joint Services 23460 Capsulorrhaphy, anterior, any type; with bone block Joint Services 23462 Capsulorrhaphy, anterior, any type;with coracoid process transfer Joint Services 23465 Capsulorrhaphy, glenohumeral joint, posterior, with or without bone block Joint Services 23466 Capsulorrhaphy, glenohumeral joint, any type multi-directional instability Joint Services 23470 ARTHROPLASTY, GLENOHUMERAL JOINT; HEMIARTHROPLASTY ARTHROPLASTY, GLENOHUMERAL JOINT; TOTAL SHOULDER [GLENOID