Effect of Weather Conditions on the Spring Migration of Eurasian Woodcock and Consequences for Breeding
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ohio Birding Checklist with Difficulty Codes Daily/Year/Life
Ohio Birding Checklist with ____ Common Merganser 2 ____ Belted Kingfisher 1 ____ Louisiana Waterthrush 2 ____ Red-breasted Merganser 1 ____ Red-headed Woodpecker 2 ____ Northern Waterthrush 2 Difficulty Codes ____ Ruddy Duck 1 ____ Red-bellied Woodpecker 1 ____ Blue-winged Warbler 1 ____ Wild Turkey 2 ____ Yellow-bellied Sapsucker 2 ____ Black-and-white Warbler 1 Daily/Year/Life ________________ ____ Pied-billed Grebe 1 ____ Downy Woodpecker 1 ____ Prothonotary Warbler 2 ____ Horned Grebe 1 ____ Hairy Woodpecker 1 ____ Tennessee Warbler 2 This checklist is arranged in three sections, each ____ Rock Pigeon 1 ____ Northern Flicker 1 ____ Nashville Warbler 1 corresponding to a pair of difficulty codes. The ____ Mourning Dove 1 ____ Pileated Woodpecker 1 ____ Kentucky Warbler 2 six codes/descriptions are based primarily on the ____ Yellow-billed Cuckoo 2 ____ American Kestrel 1 ____ Common Yellowthroat 1 collective experience of Greg Miller and Dan ____ Common Nighthawk 2 ____ Eastern Wood-Pewee 2 ____ Hooded Warbler 1 Sanders. Overall abundance and ease of ____ Chimney Swift 1 ____ Acadian Flycatcher 2 ____ American Redstart 1 identification have been factored into each of the ____ Ruby-throated Hummingbird 1 ____ Willow Flycatcher 2 ____ Cape May Warbler 2 six codes and are as follows: ____ Virginia Rail 2 ____ Least Flycatcher 1 ____ Cerulean Warbler 2 ____ Sora 1 ____ Eastern Phoebe 1 ____ Northern Parula 2 Easier to See Birds ____ Common Gallinule 2 ____ Great Crested Flycatcher 1 ____ Magnolia Warbler 1 1. Readily found within expected habitat ____ American Coot 1 ____ Eastern Kingbird 1 ____ Bay-breasted Warbler 2 during at least one season of the year. -

Red and Fallow Deer Determine the Density of Ixodes Ricinus Nymphs Containing Anaplasma Phagocytophilum
Red and Fallow Deer Determine the Density of Ixodes Ricinus Nymphs Containing Anaplasma Phagocytophilum Katsuhisa Takumi ( [email protected] ) Centre for Zoonoses and Environmental Microbiology Centre for Infectious Disease Control National Institute for Public Health and the Environment (RIVM) Bilthoven The Netherlands Tim Hofmeester Swedish University of Agricultural Sciences Faculty of Natural Resources and Agricultural Sciences Hein Sprong Centre for Zoonoses and Environmental Microbiology Centre for Infectious Disease Control National Institute for Public Health and the Environment (RIVM) Bilthoven The Netherlands Research Keywords: Ixodes ricinus nymphs, Anaplasma phagocytophilum, phagocytophilum, anaplasmosis Posted Date: October 26th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-96286/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published on January 19th, 2021. See the published version at https://doi.org/10.1186/s13071-020-04567-4. 1 Red and fallow deer determine the density of Ixodes ricinus 2 nymphs containing Anaplasma phagocytophilum 1, 2 1 3 Katsuhisa Takumi ✉, Tim R. Hofmeester , and Hein Sprong 4 1 Centre for Zoonoses and Environmental Microbiology Centre for Infectious Disease Control 5 National Institute for Public Health and the Environment (RIVM) Bilthoven The Netherlands 6 2 Department of Wildlife Fish and Environmental Studies Swedish University of Agricultural 7 Sciences Skogsmarksgränd 7 907 36 Umeå Sweden 8 ✉ Correspondence: Katsuhisa Takumi <[email protected]> 9 1 10 Abstract 11 Background: The density of Ixodes ricinus nymphs infected with Anaplasma phagocytophilum 12 is one of the parameters that determines the risk for humans and domesticated animals to 13 contract anaplasmosis. -

Migration Timing, Routes, and Connectivity of Eurasian Woodcock Wintering in Britain and Ireland
Migration Timing, Routes, and Connectivity of Eurasian Woodcock Wintering in Britain and Ireland ANDREW N. HOODLESS,1 Game & Wildlife Conservation Trust, Burgate Manor, Fordingbridge, Hampshire SP6 1EF, UK CHRISTOPHER J. HEWARD, Game & Wildlife Conservation Trust, Burgate Manor, Fordingbridge, Hampshire SP6 1EF, UK ABSTRACT Migration represents a critical time in the annual cycle of Eurasian woodcock (Scolopax rusticola), with poten- tial consequences for individual fitness and survival. In October–December, Eurasian woodcock migrate from breeding grounds in northern Eurasia over thousands of kilometres to western Europe, returning in March–May. The species is widely hunted in Europe, with 2.3–3.5 million individuals shot per year; hence, an understanding of the timing of migra- tion and routes taken is an essential part of developing sustainable flyway management. Our aims were to determine the timing and migration routes of Eurasian woodcock wintering in Britain and Ireland, and to assess the degree of connec- tivity between breeding and wintering sites. We present data from 52 Eurasian woodcock fitted with satellite tags in late winter 2012–2016, which indicate that the timing of spring departure varied annually and was positively correlated with temperature, with a mean departure date of 26 March (± 1.4 days SE). Spring migration distances averaged 2,851 ± 165 km (SE), with individuals typically making 5 stopovers. The majority of our sample of tagged Eurasian woodcock migrated to breeding sites in northwestern Russia (54%), with smaller proportions breeding in Denmark, Scandinavia, and Finland (29%); Poland, Latvia, and Belarus (9.5%); and central Russia (7.5%). The accumulated migration routes of tagged individ- uals suggest a main flyway for Eurasian woodcock wintering in Britain and Ireland through Belgium, the Netherlands, and Germany, and then dividing to pass through the countries immediately north and south of the Baltic Sea. -

Review of Illegal Killing and Taking of Birds in Northern and Central Europe and the Caucasus
Review of illegal killing and taking of birds in Northern and Central Europe and the Caucasus Overview of main outputs of the project The information collated and analysed during this project has been summarised in a variety of outputs: 1. This full report Presenting all the aspects of the project at regional and national levels http://www.birdlife.org/illegal-killing 2. Scientific paper Presenting results of the regional assessment of scope and scale of illegal killing and taking of birds in Northern and Central Europe and the Caucasus1 https://www.cambridge.org/core/journals/bird-conservation-international 3. Legislation country factsheets Presenting a review of national legislation on hunting, trapping and trading of birds in each assessed country http://www.birdlife.org/datazone/country (under ‘resources’ tab) 4. ‘The Killing 2.0’ Layman’s report Short communications publication for publicity purposes with some key headlines of the results of the project and the previous one focussing on the Mediterranean region http://www.birdlife.org/illegal-killing Credits of front cover pictures 1 2 3 4 1 Hen harrier Circus cyaneus © RSPB 2 Illegal trapping of Hen Harrier in the UK © RSPB 3 Common Coot (Fulica atra) © MISIK 4 Illegal trade of waterbirds illegally killed in Azerbaijan © AOS Citation of the report BirdLife International (2017) Review of illegal killing and taking of birds in Northern and Central Europe and the Caucasus. Cambridge, UK: BirdLife International. 1 Paper in revision process for publication in Bird Conservation International in October 2017 when this report is released 1 Executive Summary The illegal killing and taking of wild birds remains a major threat on a global scale. -

Survival Rates of Russian Woodcocks
Proceedings of an International Symposium of the Wetlands International Woodcock and Snipe Specialist Group Survival rates of Russian Woodcocks Isabelle Bauthian, Museum national d’histoire naturelle, Centre de recherches sur la biologie des populations d’oiseaux, 55 rue Buffon, 75005 Paris, France. E-mail: [email protected] Ivan Iljinsky, State University of St Petersburg, Russia. E-mail: [email protected] Sergei Fokin, State Informational-Analytical Center of Game Animals and Environment Group. Woodcock, Teterinsky Lane, 18, build. 8, 109004 Moscow, Russia. E-mail: [email protected] Romain Julliard, Museum national d’histoire naturelle, Centre de recherches sur la biologie des populations d’oiseaux, 55 rue Buffon, 75005 Paris, France. E-mail: [email protected] François Gossmann, Office national de la chasse et de la faune sauvage, 53 rue Russeil, 44 000 Nantes, France. E-mail: [email protected] Yves Ferrand, Office national de la chasse et de la faune sauvage, BP 20 - 78612 Le-Perray-en-Yvelines Cedex, France. E-mail: [email protected] We analysed 324 recoveries from 2,817 Russian Woodcocks ringed as adult or yearling in two areas in Russia (Moscow and St Petersburg). We suspected that birds belonging to these two areas may experience different hunting pressure or climatic conditions, and thus exhibit different demographic parameters. To test this hypothesis, we analysed spatial and temporal distribution of recoveries, and performed a ringing-recovery analysis to estimate possible survival differences between these two areas. We used methods developed by Brownie et al. in 1985. We found differences in temporal variations of the age ratio between the two ringing areas. -

Alpha Codes for 2168 Bird Species (And 113 Non-Species Taxa) in Accordance with the 62Nd AOU Supplement (2021), Sorted Taxonomically
Four-letter (English Name) and Six-letter (Scientific Name) Alpha Codes for 2168 Bird Species (and 113 Non-Species Taxa) in accordance with the 62nd AOU Supplement (2021), sorted taxonomically Prepared by Peter Pyle and David F. DeSante The Institute for Bird Populations www.birdpop.org ENGLISH NAME 4-LETTER CODE SCIENTIFIC NAME 6-LETTER CODE Highland Tinamou HITI Nothocercus bonapartei NOTBON Great Tinamou GRTI Tinamus major TINMAJ Little Tinamou LITI Crypturellus soui CRYSOU Thicket Tinamou THTI Crypturellus cinnamomeus CRYCIN Slaty-breasted Tinamou SBTI Crypturellus boucardi CRYBOU Choco Tinamou CHTI Crypturellus kerriae CRYKER White-faced Whistling-Duck WFWD Dendrocygna viduata DENVID Black-bellied Whistling-Duck BBWD Dendrocygna autumnalis DENAUT West Indian Whistling-Duck WIWD Dendrocygna arborea DENARB Fulvous Whistling-Duck FUWD Dendrocygna bicolor DENBIC Emperor Goose EMGO Anser canagicus ANSCAN Snow Goose SNGO Anser caerulescens ANSCAE + Lesser Snow Goose White-morph LSGW Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Intermediate-morph LSGI Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Blue-morph LSGB Anser caerulescens caerulescens ANSCCA + Greater Snow Goose White-morph GSGW Anser caerulescens atlantica ANSCAT + Greater Snow Goose Intermediate-morph GSGI Anser caerulescens atlantica ANSCAT + Greater Snow Goose Blue-morph GSGB Anser caerulescens atlantica ANSCAT + Snow X Ross's Goose Hybrid SRGH Anser caerulescens x rossii ANSCAR + Snow/Ross's Goose SRGO Anser caerulescens/rossii ANSCRO Ross's Goose -

Iceland Trip Report 2015
Iceland Trip Report May 27 to June 5, 2015 (10 days) Trip report compiled by Tour Leader Gaukur Hjartarson Iceland May & June 2015 Tour Summary May 27: The tour leader picked up all five participants at Keflavík International Airport at noon. The tour started with a known rarity at Keflavík, namely an adult drake White-winged Scoter, on the way from the pick-up site to a nearby restaurant for lunch. After lunch we scanned the Garðskagi peninsula near the international airport at Keflavík. We picked up many of the common local birds, including several Manx Shearwaters and a Purple Sandpiper. Luckily we also discovered two vagrants near Garður and had good views of a beautiful male Lapland Longspur and a Canada Goose. We then continued birding along the road to our first accommodation at Borgarnes. At Akranes we managed to see a vagrant Common Swift that had been seen there for some days. After finishing dinner at our hotel we made an evening tour to search for Eurasian Woodcocks. On the way to the site we saw a distant adult White-tailed Eagle and some Common Shelducks. We then managed to find a single Woodcock at an expected site despite some extremely cold weather. On the first day we saw a total of 45 species of birds. Photo 1. White-winged Scoter, Keflavík. Photo: Merilyn Browne. May 28: On the second day we left Borgarnes and drove to the Snæfellsnes peninsula. The day started very well with good views of an immature White-tailed Eagle near the road at Mýrar. -

Eurasian Woodcock Scolopax Rusticola
Key concepts of Article 7(4): Version 2008 Species no. 50: Eurasian Woodcock Scolopax rusticola Distribution: The Eurasian Woodcock has an extensive Palaearctic distribution. It breeds from the Azores and Ireland to the pacific coast of Russia. Birds winter in Europe, North Africa, the Middle East, India and Southeast Asia to Japan. In Europe breeding occurs from Fennoscandia and Russia to the Mediterranean basin and the Canary Islands. Movements: Breeding populations in western maritime countries are sedentary, whilst those elsewhere are migratory. Scandinavian populations move southwest to winter mainly in Britain and France. Finnish birds move mainly south and winter mostly in Italy and the Balkans. Autumn movements start after the onset of frosts. Birds from the large Russian population are recorded wintering across most of West and Central Europe. The majority of birds are in their winter quarters by November but further (sometimes large scale) movements may occur in response to cold weather. Return migration starts in February in the Mediterranean region and the first half of March elsewhere. Population size and trends: The Woodcock is a difficult bird to count accurately. The European breeding population is estimated at 1,800,000 – 6,600,000 pairs of which 1,200,000 - 5,000,000 breeds in Russia (BirdLife Int. 2004A). The EU 27 population is estimated at 460,000 - 1,500,000 pairs (BirdLife Int. 2004A, 2004B). During 1990-2000 trends were stable across most of its European range, but declining in its Russian stronghold resulting in an overall moderate decline in Europe (>10%) (BirdLife Int. 2004A). Biological and behavioural aspects: Breeding: clutch size is usually 4 eggs (2-5); incubation 21-24 days; fledging period 15-20 days but sometimes able to get off ground at 10 days; independence: 5-6 weeks after hatching; brood: normally one brood. -

Etc 2015 Award Winners
10. ETC ® Oulu / Finland, May 11 – 17, 2015 Winners List (all awards and special awards): Novice Division (Best of Division Places): 1. Sandie Miransky (Denmark), Category NB, Rook ( Corvus frugilegus ) 2. Marko Väkiparta (Finland), Category NG, White-tailed Deer ( Odocoileus virginianus ) 3. Marko Väkiparta (Finland), Category NM, Lynx ( Lynx lynx ) Professional Division (Best of Category Places): (there must be at least 90 out of 100 points achieved, so it can happen that in some categories not all or even no places were given out) Category PB2 „Medium Birds“ 1. Roland Csernák (Hungary), Eurasian Woodcock ( Scolopax rusticola ) 2. Narvydas Stankevicius (Lithuania), Mandarin Duck ( Aix galericulata ) Category PB4 „Bird Groups“ 1. Jaap van den Berg (Netherlands), 2 Garganeys ( Anas querquedula ) Category PM2 „Medium Mammals“ 1. Darius Dauksa (Lithuania), Crested Porcupine ( Histrix cristata ) Category PG1 „Small Game Heads“ 1. Jaakko Tauriainen (Finland), Red Fox ( Vulpes vulpes ) Category PG3 „Large Game Heads“ 1. Kestutis Bybartas (Lithuania), Moose ( Alces alces ) 2. Ivan Prokaza (Latvia), Wild Boar ( Sus scrofa ) Category PRA1 „Reptiles/Amphibians“ 1. Tom Hoogesteger (Finland), Massasauga Rattlesnake (Sistrurus catenatus ) Masters Division (Best of Europe Titles): (there must be at least 90 out of 100 points achieved, so it can happen that in some categories not all or even no titles were given out) Birds „Best of Europe” Jürgen Fiebig (Germany), MB4 Helmeted Curassow (Pauxi pauxi ) „Second of Europe“ Robert Stein (Germany), MB3 Southern Cassowary ( Casuarius casuarius ) „Third of Europe“ Zsombor Pünkösti (Hungary), MB1 Blue-winged Siva (Siva cyanouroptera ) Mammals „Best of Europe” Kestutis Bybartas (Lithuania), MM4 Siberian Roe Deer (Capreolus pygargus ) Reconstructions/Scientific Models „Best of Europe“ Bo Wessman (Sweden), Zander (Sander lucioperca ) Collective Artists Division: 1. -
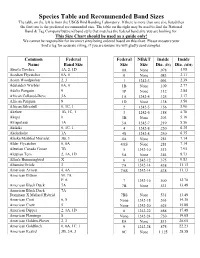
Species Table and Recommended Band Sizes the Table on the Left Is from the USGS Bird Banding Laboratory
Species Table and Recommended Band Sizes The table on the left is from the USGS Bird Banding Laboratory. If there is more than one size listed then the first one is the preferred recommended size. The table on the right may be used to find the National Band & Tag Company butt-end band style that matches the federal band size you are looking for. This Size Chart should be used as a guide only! We cannot be responsible for incorrect sizes being ordered based on this chart. Please measure your bird’s leg for accurate sizing, if you are unsure we will gladly send samples. Common Federal Federal NB&T Inside Inside Name Band Size Size Size Dia. (IN) Dia. (MM) Abert's Towhee 1A, 2, 1D 0A None .078 1.98 Acadian Flycatcher 0A, 0 0 None .083 2.11 Acorn Woodpecker 2, 3 1 1242-3 .094 2.39 Adelaide's Warbler 0A, 0 1B None .109 2.77 Adelie Penguin 9 1P None .112 2.84 African Collared-Dove 3A 1A 1242-4 .125 3.17 African Penguin 9 1D None .138 3.50 African Silverbill 0, 1C, 1 2 1242-5 .156 3.96 Akekee 1B, 1C, 1 3 1242-6 .188 4.78 Akepa 0 3B None .203 5.16 Akiapolaau 1A 3A 1242-7 .219 5.56 Akikiki 0, 1C, 1 4 1242-8 .250 6.35 Akohekohe 1A 4S 1242-8 .250 6.35 Alaska Marbled Murrelet 3B, 3 4A None .281 7.14 Alder Flycatcher 0, 0A 4AS None .281 7.14 Aleutian Canada Goose 7B 5 1242-10 .313 7.95 Aleutian Tern 2, 1A, 1D 5A None .344 8.73 Allen's Hummingbird X 6 1242-12 .375 9.53 Altamira Oriole 3 7A 1242-14 .438 11.13 American Avocet 4, 4A 7AS 1242-14 .438 11.13 American Bittern M: 7A F: 6 7 1242-16 .500 12.70 American Black Duck 7A 7B None .531 13.49 American -

Survey of Wintering Eurasian Woodcock in Western Europe
Survey of Wintering Eurasian Woodcock in Western Europe DAVID GONÇALVES,1 CIBIO-InBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Campus Agrário de Vairão, 4485-661 Vairão, Portugal and Departamento de Biologia, Faculdade de Ciências, Universidade do Porto, R. Campo Alegre s/n, 4169-007 Porto, Portugal TIAGO M. RODRIGUES, Departamento de Biologia, Faculdade de Ciências, Universidade do Porto, R . Campo Alegre s/n, 4169-007 Porto, Portugal and Direção Regional dos Recursos Florestais, Rua do Contador, 23, 9500-050 Ponta Delgada, Portugal PAOLO PENNACCHINI, Féderation des Associations Nationales des Bécassiers du Palearctique Occidental (FANBPO), Via Fausto Vagnetti 12, 52031 Anghiari, Italy JEAN-PIERRE LEPETIT, Club National des Bécassiers (CNB), 3 , Allée des chénes, 87220 Feytiat, France LARRY TAAFFE, The National Woodcock Association of Ireland (NWAOI), Effoldstown House, Lusk, Co. Dublin, Ireland MARCO TUTI, Beccacciai d’Italia (BDI), C lemente Rebora 14 - 73100 Lecce, Italy BRUNO MEUNIER, Club National des Bécassiers (CNB), 105, Rue Louis Pergaud, Villeneuve, 16430 Champniers, France JEAN-PIERRE CAMPANA,2 Club National des Bécassiers (CNB), 8 , Impasse des Bois, La Granje À Robin, 17770 Aumagne, France GIANLUIGI GREGORI, Club della Beccaccia (CDB), L oc. Morani di sotto, 22 – 61040 Mercatello sul Metauro (PU), Italy ALBERTO PELLEGRINI, Club della Beccaccia (CDB), L oc. Morani di sotto, 22 – 61040 Mercatello sul Metauro (PU), Italy GIUSEPPE RAHO, Beccacciai d’Italia (BDI), Clemente Rebora 14 - 73100 Lecce, Italy PAUL DUCHEIN, Association Suisse des Bécassiers (ASB), P lanafaye 118, CH - 1752 Villars sur Glâne, Switzerland COLIN TROTMAN, Welsh Woodcock Club (WWC), Department of Adult Continuing Education (DACE), K eir Hardie Building, Swansea University, Singleton Park, Swansea, SA2 8PP, Wales, UK MIGUEL MINONDO, Club de Cazadores de Becada (CCB), A venida Schulz 8, 4°Dcha. -

Influence of Weather on the Eurasian Woodcock's Breeding Display
Influence of Weather on the Eurasian Woodcock’s Breeding Display CHRISTOPHER J. HEWARD,1 Game & Wildlife Conservation Trust, Burgate Manor, Fordingbridge, SP6 1EF. UK ANDREW LOWE, Birklands Ringing Group, 159 Sherwood Street, Market Warsop, Mansfield, NG20 0JX. UK GREG J. CONWAY, British Trust for Ornithology, The Nunnery, Thetford, Norfolk, IP24 2PU. UK ANDREW N. HOODLESS, Game & Wildlife Conservation Trust, Burgate Manor, Fordingbridge, SP6 1EF. UK ABSTRACT: Counts of displaying male Eurasian woodcock (Scolopax rusticola) form the basis for breeding Eurasian woodcock surveys in many regions across Europe and provide the only practical method of assessing the species’ abun- dance. This paper investigates the effect that weather may have on the results of these surveys, principally considering its influence upon Eurasian woodcock display behavior and detectability by surveyors. We assessed data from an annual Eurasian woodcock survey conducted in the Britain during 2004–2015 and correlated them with a number of weather vari- ables. This is supplemented by tracking data gathered from 19 male Eurasian woodcock to assess how weather might affect each individual’s decision to display. We found that counts of roding Eurasian woodcock were positively related to the amount of rainfall in the 2 weeks preceding the survey and negatively related to wind speed on the evenings that surveys were conducted. The likelihood that tagged male Eurasian woodcock displayed decreased in relation to wind speed and increased in relation to minimum air temperature. To guarantee that counts of displaying males provide a representative measure of abundance, we recommend that surveys consist of at ≥3 visits to each site within each year, that visits are spread as widely as possible across the peak displaying season, and that analyses are based on maximal counts rather than means to reduce the effects of surveys conducted in sub-optimal weather conditions.