Full Text in Pdf Format
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Istituto Scolastico Alto Malcantone
Istituto scolastico Alto Malcantone Disposizioni scolastiche 2020 - 2021 Scuole dell’infanzia di Arosio e di Cademario Scuola elementare di Cademario 1 Ai genitori delle allieve e degli allievi dell’ Istituto scolastico Alto Malcantone Introduzione Le seguenti disposizioni hanno lo scopo di dare un’informazione sulle norme che regolano il funzionamento dell’Istituto scolastico Alto Malcantone composto dalle sedi di Scuola dell’infanzia di Cademario e Arosio e dalla sede di scuola elementare di Cademario. Esse vogliono costituire una premessa su cui costruire un buon rapporto tra scuola e famiglia. L’attenersi a queste disposizioni durante l’intero anno scolastico rientra nell’ambito di un discorso educativo che coinvolge tutte le componenti della scuola. Ognuno deve quindi impegnarsi per rispettare, e far rispettare, queste semplici regole. 1. DISPOSIZIONI GENERALI 1.1 Calendario scolastico Le scuole dell’infanzia di Cademario e di Arosio e la scuola elementare di Cademario riapriranno lunedì 31 agosto 2020 alle ore 8.30. Periodi di vacanza: vacanze autunnali: dal 31 ottobre all’8 novembre 2020 vacanze di Natale: dal 24 dicembre 2020 al 6 gennaio 2021 vacanze di Carnevale: dal 13 febbraio al 21 febbraio 2021 vacanze di Pasqua: dal 2 aprile all’11 aprile 2021 Sono inoltre giorni di vacanza venerdì 14 maggio 2021 e venerdì 4 giugno 2021. In tutte le scuole le lezioni terminano venerdì 18 giugno 2021. Per disposizione del Collegio degli ispettori non vengono concesse deroghe al calendario scolastico. La direzione non autorizzerà quindi congedi, partenze anticipate o prolungamenti di vacanze. Le famiglie si assumono la responsabilità per eventuali assenze, le quali dovranno comunque essere comunicate al docente di classe con almeno una settimana di anticipo. -

Medio Malcantone: Fase 3
Preambolo al rapporto della Commissione per l’aggregazione dei Comuni dell’Alto Malcantone Accanto alla stesura del rapporto tecnico, si è ritenuto importante redigere un preambolo al rapporto che spiegasse le ragioni e le aspettative che hanno portato i Comuni dell’Alto Malcantone a studiare e approfondire questo progetto di aggregazione. Perché aggregare i Comuni dell’Alto Malcantone? A questa domanda la Commissione ha cercato di dare una risposta analizzando i vari aspetti che contraddistinguono la situazione attuale e le prospettive future dei cinque Comuni coinvolti. Risposta 1: Per migliorare la situazione istituzionale In tutti i Comuni, eccetto Arosio, il legislativo è rappresentato dall’Assemblea Comunale. Pur riconoscendo in questa istituzione forse la più originale espressione della democrazia diretta, tuttavia l’Assemblea comunale ha qualche limite dovuto all’impossibilità di approfondire tematiche, oggi anche molto complesse. Un Comune moderno ha la necessità di avere un organo legislativo che sia in grado di approfondire le tematiche, di farsi promotore di iniziative propositive e progettuali e di fungere da vero “controllore” dell’operato dell’esecutivo. E l’aggregazione permetterà appunto di dotare il nuovo Comune di un Consiglio Comunale, formato in modo da tenere in considerazione le realtà locali e affiancato dalle cosiddette assemblee di quartiere dove potranno essere coinvolti la popolazione, i patriziati e le parrocchie. Il coinvolgimento della popolazione potrebbe anche passare attraverso l’iniziativa “Agenda 21” già promossa con successo in altri Comuni ticinesi. Per quanto riguarda gli Esecutivi, in alcuni casi, da qualche quadriennio, i Municipali sono eletti in forma tacita e si fatica a sostituire eventuali partenti. -
Sentiero Del Castagno
Castagno2007 TEDESCO imp:Castagno2006 TEDESCO imp 27.3.2007 13:09 Pagina 13 Ente Turistico del Luganese Der periodische Grasschnitt sollte mindestens zweimal Einmal am Boden, sollten die Früchte so früh wie möglich DieZeitderSchlägebeginnt.DerNiederwald(palina)istdie 0IAZZA3TAZIONE #( 4ESSERETE während der Vegetationsperiode durchgeführt werden. aufgelesen werden, um zu vermeiden, dass sie von Pilzen Betriebsart der Kastanienwälder welche eine Hauptproduk- 4 p& Eine optimale Alternative zu dieser kostspieligen Tätigkeit oder Insekten befallen werden. Der gute Erfolg dieser Hand- tion von Brennholz erlaubt: es handelt sich um Wälder, die WWWLUGANOTURISMOCHpTESSERETE LUGANOTURISMOCH ist die Viehbeweidung lung wird von der Beachtung bestimmter Bedingungen ab- sich vegetativ vermehren (Stockausschläge) mit 15-20 Herbst Die lichte Struktur der Deckung und das Vorhandensein hängig gemacht, die die Verluste an Produkte auf das Mini- Winter Jahre turnus. Um Qualitätsholz und stabilere Bäume zu er- Sommer von alten und eindrucksvollen Bäumen tragen der Er- mum einschränken. In der Regel ist das Sammeln bis ein zeugen ist es unbedingt unentbehrlich, den Schnitt des höhung der ökologischen Bedeutung der Selven bei. Letz- bestimmtesDatumdemBesitzervorbehalten(imMalcanto- Stockes bodeneben und mit einer glatten und schiefen tere übernehmen vielfältige biologische Funktionen und ne üblicherweise bis San Martino, 11 November): nach die- Schnittoberfläche auszuführen. Was die Selve betrifft, ist stellen somit ein reiches Ökosystem dar. sem Datum ist das Sammeln für alle erlaubt. Wie für jede die Erziehung-, Produktion- oder Nachholstutzung im Fall Subsistenzkultur,auch in der Produktion der Kastanien ent- von Krebsbefall oder Vernachlässigung die während dieser wickelte sich eine Differenzierung des Produktes in viele Jahreszeit wichtigste auszuführende Arbeit. unterschiedliche Sorten, nach der Dauer der Reifung, der Nutzungsart oder dem Verbreitungsgebiet. -

Scheda Maroggia
MAROGGIA Altitudine (m s.l.m.): 276 Piramide delle età, secondo il sesso Elezione del Gran Consiglio 2019: schede Superficie (km2): 0,99 2 Densità popolazione (ab./km ): 707,07 Uomini PLR: 19,0% PS: 14,9% Municipali: 5 Donne LEGA: 14,0% SSI: 19,0% Consiglieri comunali: 20 PPD: 12,4% Altri: 20,7% Regione: Mendrisiotto 90 e + 80-89 Distretto: Lugano 70-79 Carattere urbano: agglomerato di 60-69 Lugano, comune della cintura 50-59 Riferimento cartina: 63 40-49 30-39 20-29 10-19 0-9 2520 1510 5 05 10152025 % Tasso di partecipazione: 63,1% Aziende, secondo la classe dimensionale (in addetti) % Meno di 5 Da 5 a meno di 10 Da 10 a meno di 50 50 e più 72,8 16,3 10,9 Ass. In % Ass. In % Popolazione 700 100,0 Aziende 92 100,0 Uomini 355 50,7 Settore primario 1 1,1 Donne 345 49,3 Settore secondario 12 13,0 Settore terziario 79 85,9 Svizzeri 452 64,6 Stranieri 248 35,4 Addetti 344 100,0 Settore primario 1 0,3 0-19 anni 119 17,0 Settore secondario 85 24,7 20-64 anni 415 59,3 Settore terziario 258 75,0 65 e più anni 166 23,7 Uomini 223 64,8 Celibi/nubili 305 43,6 Donne 121 35,2 Coniugati 290 41,4 Divorziati 68 9,7 Settore alberghiero Vedovi 37 5,3 Stabilimenti 1 ... Pernottamenti e tasso di occupazione X X Nascite 10 ... Finanze pubbliche Edifici esclusivamente abitativi 134 100,0 Moltiplicatore d'imposta 85 .. -

Comune Di Alto Malcantone
COMUNE DI ALTO MALCANTONE CONSIGLIO COMUNALE RIASSUNTO VERBALE DELLE DISCUSSIONI della seduta ordinaria del Consiglio comunale mercoledì 22 luglio 2020, ore 20.30 ORDINE DEL GIORNO: 1. Appello nominale 2. Approvazione dell’ordine del giorno 3. Approvazione verbale (riassunto della discussione) della seduta ordinaria del 23 gennaio 2020 4. Rinnovo dell’Ufficio presidenziale del Consiglio comunale - Presidente - vice Presidente - 2 scrutatori 5. Completazione Commissione della gestione (nomina del sostituto di Sandro Poncini) 6. Messaggio municipale n°232 accompagnante le dimissioni dalla carica di Consigliere comunale di Massimo Gianoli 7. Messaggio municipale n° 226 accompagnante la richiesta di aggiornamento del preventivo 2019 per la contabilizzazione di un ammortamento supplementare di CHF 745'208.35 a carico della gestione comunale 2019 8. Messaggio municipale n° 227 accompagnante il conto consuntivo 2019 dell’Amministrazione comunale di Alto Malcantone 9. Messaggio municipale n°223 concernente l’adozione delle varianti del Piano Regolatore - sezione di Arosio, del Comune di Alto Malcantone 10. Messaggio municipale n°224 concernente la richiesta di un credito di CHF 69'000.00 per l’allestimento di un nuovo Percorso Vita a Vezio 11. Messaggio municipale n° 225 concernente la proposta di accettazione del lascito Tami da parte dell’assemblea della già Associazione dei Comuni Regione Malcantone 12. Messaggio municipale n° 228 accompagnante la richiesta di un credito di CHF 70'000.00 per l’aggiornamento offline della banca dati Ge.Co.Ti, l’accompagnamento alla pubblicazione e l’evasione dei ricorsi nell’ambito dell’emissione dei contributi provvisori di costruzione sulle opere di canalizzazione PGS sull’intero comprensorio comunale 13. -

Angelo Rossi, Lo Sviluppo Della Regione Urbana Del Luganese
Lo sviluppo della regione urbana del Luganese nell’era della globalizzazione e della metropolizzazione Angelo Rossi Lo sviluppo della regione urbana del Luganese nell’era della globalizzazione e della metropolizzazione COMMISSIONE REGIONALE DEI TRASPOrtI DEL LUGANESE Si ringraziano sentitamente per il loro contributo: ASPAN-TI (Associazione svizzera per la pianificazione del territorio, Gruppo regionale ticinese), Canobbio Ferrovie Luganesi SA, Lugano Indirizzo dell’autore: Angelo Rossi, “Trends and Bends”, Lettenring 33, 8114 Dänikon © 2008 . Commissione regionale dei trasporti del Luganese (CRTL) via Sala 13, CH-6963 Pregassona (Lugano) ISBN 978-88-95679-11-2 Indice Presentazione della Commissione regionale dei trasporti del Luganese 1 Per chi deve leggere in fretta 3 Capitolo I: Le tendenze di fondo 13 1.1 Dalla globalizzazione alla metropolizzazione 14 1.2 L’urbanizzazione in Europa nell’era del policentrismo 16 1.3 Le nuove tendenze dell’urbanizzazione in Svizzera 18 1.3.1 La città come soggetto di politica nazionale 19 1.3.2 La visione prospettica 20 1.3.3 La riforma istituzionale 27 1.4 Lo sviluppo urbano in Ticino 29 1.4.1 L’evoluzione degli agglomerati urbani 30 1.4.2 Gli aspetti territoriali dello sviluppo urbano 32 1.4.3 La dinamica economica e lo sviluppo urbano 35 1.5 Che cosa significano queste tendenze per la regione urbana di Lugano? 39 1.5.1 La globalizzazione e lo sviluppo della struttura di produzione 39 1.5.2 La globalizzazione e lo sviluppo territoriale 40 Capitolo II: Lo sviluppo secolare della regione urbana di Lugano -
Sred Newsletter Q4 2020
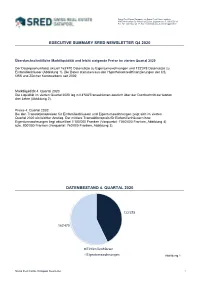
Swiss Real Estate Datapool, c/o Swiss Real Estate Institute, HWZ Hochschule für Wirtschaft Zürich, Lagerstrasse 5, 8004 Zürich Tel. +41 (0)43 322 26 88, Fax: +41 (0)43 322 26 33, [email protected] EXECUTIVE SUMMARY SRED NEWSLETTER Q4 2020 Überdurchschnittliche Marktliquidität und leicht steigende Preise im vierten Quartal 2020 Der Datenpool umfasst aktuell 163'470 Datensätze zu Eigentumswohnungen und 122'378 Datensätze zu Einfamilienhäuser (Abbildung 1). Die Daten stammen aus den Hypothekarkreditfinanzierungen der CS, UBS und Zürcher Kantonalbank seit 2002. Marktliquidität 4. Quartal 2020: Die Liquidität im vierten Quartal 2020 lag mit 4'532Transaktionen deutlich über der Durchschnitt der letzten drei Jahre (Abbildung 2). Preise 4. Quartal 2020: Bei den Transaktionspreisen für Einfamilienhäusern und Eigentumswohnungen zeigt sich im vierten Quartal 2020 ein leichter Anstieg. Der mittlere Transaktionspreis für Einfamilienhäusern bzw. Eigentumswohnungen liegt aktuell bei 1'100'000 Franken (Vorquartal: 1'040'000 Franken, Abbildung 4) bzw. 800'000 Franken (Vorquartal: 760'000 Franken, Abbildung 3). DATENBESTAND 4. QUARTAL 2020 122'378 163'470 Einfamilienhäuser Eigentumswohnungen Abbildung 1 Swiss Real Estate Datapool Newsletter MARKTÜBERSICHT SCHWEIZ LIQUIDITÄT Anzahl Transaktionen nach Objektart 5'000 4'000 3'000 2'000 1'000 0 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 2017 2018 2019 2020 Eigentumswohnungen Einfamilienhäuser Total Abbildung 2 PREISENTWICKLUNG Eigentumswohnungen 1'250'000 1'000'000 750'000 500'000 250'000 0 Q1 Q2 Q3 Q4 Q1 -

Melody of Dharma
Melody of Dharma An Introductory Teaching on Taking Refuge Buddha Nature by His Holiness the Sakya Trizin A teaching by H.E. Ratna Vajra Rinpoche Global Ecological Crisis: An Aspirational Prayer by His Holiness the Sakya Trizin A Publication of the Office of Sakya Dolma Phodrang Dedicated to the Dharma Activities of His Holiness the Sakya Trizin 2010 • No.3 CONTENTS 3 From The Editors 5 Chogye Trichen Rinpoche / Nalendra Monastery 6 Remembering Great Masters –– Virupa 8 Virupa's Dohakosa 14 Global Ecological Crisis: An Aspirational Prayer –– by His Holiness the Sakya Trizin 16 Taking Refuge –– A teaching by His Holiness the Sakya Trizin (part 2) 22 Buddha Nature –– A teaching by H.E. Ratna Vajra Rinpoche 28 News From The Phodrang: Dungsey Akasha Rinpoche 29 The Khön Family 32 Dharma Activities 32 • His Holiness' Visit to Arunachal Pradesh 34 • His Holiness’ Russian and European Teaching Tour 46 • Lamdre in Kuttolsheim 57 Mahavairocana Puja –– A brief History by His Holiness the Sakya Trizin 59 Summer Retreat 59 His Holiness' Retreat House Publisher: The Office of Sakya Dolma Phodrang Editing Team: Executive Editor: Ani Jamyang Wangmo Mrs. Yang Dol Tsatultsang (Dagmo Kalden’s mother), Managing Editor: Patricia Donohue Danijela Stamatovic, Sherab Chöden , Supervising Editor: Terri Lee Rosemarie Hemscheidt Art Director/Designer: Chang Ming-Chuan Cover Photo: Nalendra Monastery, Tibet Photographs: Cristina Vanza From The Editors We are very pleased to welcome our readers to this, our third issue of Melody of Dharma, with a particularly warm greeting to our new subscribers. We wish to extend our heartfelt thanks to our sponsors, whose generosity made this issue possible, and to all those who have contributed to the magazine in one way or another. -

Foglioufficiale 56 2017
Si pubblica Repubblica e Bellinzona il martedì e il venerdì Cantone Ticino Venerdì 56 14 luglio 2017 www.ti.ch/fu Anno 174 2017 Cancelleria dello Stato Oggi con il Bollettino ufficiale Foglioufficiale delle leggi Atti legislativi e dell’Amministrazione BU 6225 1 Atti dello stato civile – 2 Atti ed avvisi giudiziari 6245 3 Atti ed avvisi d’esecuzioni e fallimenti 6252 4 Atti ed avvisi comunali, patriziali, parrocchiali e consortili 6301 5 Atti diversi 6320 6 Iscrizioni nel Registro di commercio 6325 7 Gli avvisi per la parte interna Gli annunci pubblicitari Richieste amministrative e abbonamenti devono pervenire al più tardi alle ore 11:00 sono da indirizzare esclusivamente a da inoltrare a di lunedì o giovedì all’indirizzo e-mail Publicitas SA Cancelleria dello Stato [email protected] Via Senago 42, 6915 Pambio Noranco Servizio di Segreteria del Consiglio di Stato telefono gratuito 0800 144 349 telefono 091 910 35 65 Amministrazione del Foglio ufficiale fax 091 910 35 49 Tariffe Piazza Governo 6, 6501 Bellinzona e-mail: [email protected] Linea di 1 mm di altezza e 103 di larghezza telefono 091 814 43 49 Tariffe Avvisi ufficiali fr. 1.55 (+IVA 8% fr. 1.67 x mm) fax 091 814 44 01 1/1 pagina fr. 795.– / a colori fr. 950.– Avvisi diversi fr. 1.95 (+IVA 8% fr. 2.10 x mm) e-mail: [email protected] 1/2 pagina fr. 415.– / a colori fr. 515.– Tariffe e abbonamenti 1/4 pagina fr. 220.– / a colori fr. 260.– Svizzera IVA 2,5% compresa 1/8 pagina fr. -
Sentiero Del Castagno
Ente turistico del Malcantone CH-6987 Caslano – Piazza Lago Telefono0916062986 – Fax 0916065200 www.malcantone.ch E-mail: [email protected] Arosio Mugena Vezio Sentiero Fescoggia Arosio del castagno Kastanienweg Auf der Entdeckung des Alto Malcantone: Land der Kastanien «S’u piöv ur dì d’Ascension Ab dem Grotto Sgambada in Arosio erreicht man zuerst u fa castegn fin i boscûn» den Kastanienhain von Induno und dann weiter die Kirche von San Michele. In der Nähe befindet sich eine alte …regnet es am Auffahrtstag, und umgewandelte «grà» (metato). Die Exkursion führt werden sogar die dürren weiter Richtung Mugena und bietet auf dem Weg eine Äste Früchte tragen. (Astano) herrliche und aussergewöhnliche Aussicht auf die ganze Gegend des Alto Malcantone, hauptsächlich auf die Caroggio-Ebene. Nachdem man sich den Dorfkern von Mugena angesehen hat, geht es weiter Richtung «Busgnone», wo man in der Nähe eines «natürlichen Schwimmbassins» den Magliasina-Fluss überquert. Der Wanderweg führt entlang des Valle di Firinescio und somit oberhalb Vezio bis zum Dorf Fescoggia weiter, immer in Begleitung der treuen Edelkastanie. Ab Fescoggia beginnt der Rückweg hinunter zuerst Richtung Caroggio, darauffolgend hinauf zu Mugena und Arosio, immer mit Blick in die Höhe zu den schon vorher gesichteten Kastanienbäumen, die stets unbeweglich auf uns herabschauen. Der Pfad ist mit der üblichen, mit dem Symbol der Kastanie ergänzten Markierung festgesetzt. Sie sieht 8 auf dem Gelände einfach markierte didaktische Stellen vor, die dann in diesem Prospekt ausführlicher illustriert sind, um euch die wesentlichen Auskünfte zu liefern und euer Interesse für eventuelle weitere Einzelheiten anzuregen. Streusammlung in der Vergangenheit. Vergesst nicht auf dem Wanderweg alle eure zur Verfügung stehenden Sinne zu verwenden: die Sicht, der Geruch, der Tastsinn; es lohnt sich! Benötigte Wanderzeit: 5-6 Stunden ab Arosio. -

Foglio Ufficiale 8/2019 Del 25.1.2019
Si pubblica Repubblica e Bellinzona il martedì e il venerdì Cantone Ticino Venerdì 8 25 gennaio 2019 www.ti.ch/fu Anno 176 2019 n il le Cancelleria i co ficia Ogg o uf dello Stato ettin Boll i legg delle Foglio ufficial e U Atti legislativi e dell’Amministrazione B 697 1 Atti dello stato civile – 2 Atti ed avvisi giudiziari 749 3 Atti ed avvisi d’esecuzioni e fallimenti 775 4 Atti ed avvisi comunali, patriziali, parrocchiali e consortili 808 5 Atti diversi 842 6 Iscrizioni nel Registro di commercio 849 7 Gli avvisi per la parte interna Gli annunci pubblicitari Richieste amministrative e abbonamenti devono pervenire al più tardi alle ore 11 :00 sono da inoltrare a da inoltrare a di lunedì o giovedì all’indirizzo e-mail Cancelleria dello Stato Cancelleria dello Stato [email protected] Servizio di Segreteria Servizio di Segreteria del Consiglio di Stato telefono gratuito 0800 144 349 del Consiglio di Stato Amministrazione del Foglio ufficiale Tariffe Amministrazione del Foglio ufficiale Piazza Governo 6, 6501 Bellinzona Linea di 1 mm di altezza e 103 di larghezza Piazza Governo 6, 6501 Bellinzona telefono 091 814 43 49 Avvisi ufficiali fr. 1.55 (+IVA 7,7% fr. 1.67 x mm) telefono 091 814 43 49 fax 091 814 44 01 Avvisi diversi fr. 1.95 (+IVA 7,7% fr. 2.10 x mm) e-mail: [email protected] e-mail: [email protected] Tariffe Tariffe e abbonamenti 1/1 pagina fr. 795.– / a colori fr. 950. – Svizzera IVA 2,5% compresa 1/2 pagina fr. -

Ambrosia Artemisiifolia, Heracleum Mantegazzianum, Senecio Inaequidens E Sicyos Angulatus in Canton Ticino
Ambrosia artemisiifolia, Heracleum mantegazzianum, Senecio inaequidens e Sicyos angulatus in Canton Ticino RAPPORTO 2017 SERVIZIO FITOSANITARIO CANTONALE Sezione dell’agricoltura, Servizio fitosanitario, Laura Torriani ,Giorgia Mattei. Ambrosia artemisiifolia, Heracleum mantegazzianum, Senecio inaequidens e Sicyos angulatus in Ticino. Rapporto 2017 Sommario RIASSUNTO ................................................................................................................................ 3 1. INFORMAZIONE E SENSIBILIZZAZIONE .................................................................................... 4 2. SQUADRE DI INTERVENTO ...................................................................................................... 5 3. MONITORAGGIO AMBROSIA .................................................................................................. 6 3.1 FOCOLAI ........................................................................................................................................ 6 3.2 SITUAZIONE LUNGO LE AUTOSTRADE .................................................................................................. 10 3.3 STADI FENOLOGICI .......................................................................................................................... 11 3.4 CONCENTRAZIONE DEI POLLINI NELL’ARIA ............................................................................................ 11 3.5 OPHRAELLA COMMUNA ..................................................................................................................