Species Assessment for Pine Pinion Moth
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Methods and Work Profile
REVIEW OF THE KNOWN AND POTENTIAL BIODIVERSITY IMPACTS OF PHYTOPHTHORA AND THE LIKELY IMPACT ON ECOSYSTEM SERVICES JANUARY 2011 Simon Conyers Kate Somerwill Carmel Ramwell John Hughes Ruth Laybourn Naomi Jones Food and Environment Research Agency Sand Hutton, York, YO41 1LZ 2 CONTENTS Executive Summary .......................................................................................................................... 8 1. Introduction ............................................................................................................ 13 1.1 Background ........................................................................................................................ 13 1.2 Objectives .......................................................................................................................... 15 2. Review of the potential impacts on species of higher trophic groups .................... 16 2.1 Introduction ........................................................................................................................ 16 2.2 Methods ............................................................................................................................. 16 2.3 Results ............................................................................................................................... 17 2.4 Discussion .......................................................................................................................... 44 3. Review of the potential impacts on ecosystem services ....................................... -

Pinus Echinata Shortleaf Pine
PinusPinus echinataechinata shortleafshortleaf pinepine by Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia One of the most widespread pines of the Eastern United Sates is Pinus echinata, shortleaf pine. Shortleaf pine was identified and named in 1768. The scientific name means a “prickly pine cone tree.” Other common names for shortleaf pine include shortstraw pine, yellow pine, Southern yellow pine, shortleaf yellow pine, Arkansas soft pine, Arkansas pine, and old field pine. Among all the Southern yellow pines it has the greatest range and is most tolerant of a variety of sites. Shortleaf pine grows Southeast of a line between New York and Texas. It is widespread in Georgia except for coastal coun- ties. Note the Georgia range map figure. Pinus echinata is found growing in many mixtures with other pines and hardwoods. It tends to grow on medium to dry, well-drained, infertile sites, as compared with loblolly pine (Pinus taeda). It grows quickly in deep, well-drained areas of floodplains, but cannot tolerate high pH and high calcium concentrations. Compared with other Southern yellow pines, shortleaf is less demanding of soil oxygen content and essential element availability. It grows in Hardiness Zone 6a - 8b and Heat Zone 6-9. The lowest number of Hardiness Zone tends to delineate the Northern range limit and the largest Heat Zone number tends to define the South- ern edge of the range. This native Georgia pine grows in Coder Tree Grow Zone (CTGZ) A-D (a mul- tiple climatic attribute based map), and in the temperature and precipitation cluster based Coder Tree Planting Zone 1-6. -

Important Food Plants for Backyard Songbirds of the Catskills
Important Food Plants for Backyard Songbirds of the Catskills Woody Plants ****25-50%, ***10-25% of diet **5-10%, *2-5&% of diet 0.5-2% of diet Maples Box-elder Acer negundo Evening Grosbeak **** Coccothraustes vespertinus American Goldfinch Carduelis tristis Moosewood Acer pensylvanicum Purple Finch * Carpodacus purpureus Yellow-bellied Sapsucker (sap) Sphyrapicus varius Red Maple Acer rubrum Rose-breasted Grosbeak * Pheucticus ludovicianus Silver Maple Acer saccharinum Red-breasted Nuthatch * Sitta canadensis Sugar Maple Acer saccharum Mountain Maple Acer spicatum Serviceberries Downy Serviceberry Amelanchier arborea Cedar Waxwing * Bombicilla cedrorum Tufted Titmouse Baeolophus bicolor Shadblow Serviceberry Amelanchier canadensis Veery * Catharus fuscescens Northern Cardinal Cardinalis cardinalis Smooth Serviceberry Amelanchier laevis Hermit Thrush * Catharus guttatus Hermit Thrush Catharus guttatus Running Serviceberry Amelanchier stolonifera Gray Catbird * Dumetella carolinensis Northern Flicker Coraptes auratus Baltimore Oriole * Icterus galbula American Crow Corvus brachyrhyncos Blue Jay Cyanocitta cristata Wood Thrush Hylocichla mustelina Northern Mockingbird Mimus polyglottus Eastern Towhee Papilo erythrophthalmus Rose-breasted Grosbeak Pheucticus ludovicianus Downy Woodpecker Picoides pubescens Hairy Woodpecker Picoides villosus Scarlet Tanager Piranga olivacea Black-capped Chickadee Poecile atricapillus Eastern Bluebird Sialia sialis Brown Thrasher Toxostoma rufin American Robin Turdus migratorius Aralias Bristly Sarsparilla -

Xyleninae 73.087 2385 Small Mottled Willow
Xyleninae 73.087 2385 Small Mottled Willow (Spodoptera exigua) 73.089 2386 Mediterranean Brocade (Spodoptera littoralis) 73.091 2396 Rosy Marbled (Elaphria venustula) 73.092 2387 Mottled Rustic (Caradrina morpheus) 73.093 2387a Clancy's Rustic (Caradrina kadenii) 73.095 2389 Pale Mottled Willow (Caradrina clavipalpis) 73.096 2381 Uncertain (Hoplodrina octogenaria) 73.0961 2381x Uncertain/Rustic agg. (Hoplodrina octogenaria/blanda) 73.097 2382 Rustic (Hoplodrina blanda) 73.099 2384 Vine's Rustic (Hoplodrina ambigua) 73.100 2391 Silky Wainscot (Chilodes maritima) 73.101 2380 Treble Lines (Charanyca trigrammica) 73.102 2302 Brown Rustic (Rusina ferruginea) 73.103 2392 Marsh Moth (Athetis pallustris) 73.104 2392a Porter's Rustic (Athetis hospes) 73.105 2301 Bird's Wing (Dypterygia scabriuscula) 73.106 2304 Orache Moth (Trachea atriplicis) 73.107 2300 Old Lady (Mormo maura) 73.109 2303 Straw Underwing (Thalpophila matura) 73.111 2097 Purple Cloud (Actinotia polyodon) 73.113 2306 Angle Shades (Phlogophora meticulosa) 73.114 2305 Small Angle Shades (Euplexia lucipara) 73.118 2367 Haworth's Minor (Celaena haworthii) 73.119 2368 Crescent (Helotropha leucostigma) 73.120 2352 Dusky Sallow (Eremobia ochroleuca) 73.121 2364 Frosted Orange (Gortyna flavago) 73.123 2361 Rosy Rustic (Hydraecia micacea) 73.124 2362 Butterbur (Hydraecia petasitis) 73.126 2358 Saltern Ear (Amphipoea fucosa) 73.127 2357 Large Ear (Amphipoea lucens) 73.128 2360 Ear Moth (Amphipoea oculea) 73.1281 2360x Ear Moth agg. (Amphipoea oculea agg.) 73.131 2353 Flounced Rustic (Luperina -

Vulnerability of At-Risk Species to Climate Change in New York (PDF
Vulnerability of At-risk Species to Climate Change in New York Matthew D. Schlesinger, Jeffrey D. Corser, Kelly A. Perkins, and Erin L. White New York Natural Heritage Program A Partnership between The Nature Conservancy and the NYS Department of Environmental Conservation New York Natural Heritage Program i 625 Broadway, 5th Floor Albany, NY 12233-4757 (518) 402-8935 Fax (518) 402-8925 www.nynhp.org Vulnerability of At-risk Species to Climate Change in New York Matthew D. Schlesinger Jeffrey D. Corser Kelly A. Perkins Erin L. White New York Natural Heritage Program 625 Broadway, 5th Floor, Albany, NY 12233-4757 March 2011 Please cite this document as follows: Schlesinger, M.D., J.D. Corser, K.A. Perkins, and E.L. White. 2011. Vulnerability of at-risk species to climate change in New York. New York Natural Heritage Program, Albany, NY. Cover photos: Brook floater (Alismodonta varicosa) by E. Gordon, Spadefoot toad (Scaphiopus holbrookii) by Jesse Jaycox, and Black Skimmers (Rynchops niger) by Steve Young. Climate predictions are from www.climatewizard.org. New York Natural Heritage Program ii Executive summary Vulnerability assessments are rapidly becoming an essential tool in climate change adaptation planning. As states revise their Wildlife Action Plans, the need to integrate climate change considerations drives the adoption of vulnerability assessments as critical components. To help meet this need for New York, we calculated the relative vulnerability of 119 of New York’s Species of Greatest Conservation Need (SGCN) using NatureServe’s Climate Change Vulnerability Index (CCVI). Funding was provided to the New York Natural Heritage Program by New York State Wildlife Grants in cooperation with the U.S. -

Insects That Feed on Trees and Shrubs
INSECTS THAT FEED ON COLORADO TREES AND SHRUBS1 Whitney Cranshaw David Leatherman Boris Kondratieff Bulletin 506A TABLE OF CONTENTS DEFOLIATORS .................................................... 8 Leaf Feeding Caterpillars .............................................. 8 Cecropia Moth ................................................ 8 Polyphemus Moth ............................................. 9 Nevada Buck Moth ............................................. 9 Pandora Moth ............................................... 10 Io Moth .................................................... 10 Fall Webworm ............................................... 11 Tiger Moth ................................................. 12 American Dagger Moth ......................................... 13 Redhumped Caterpillar ......................................... 13 Achemon Sphinx ............................................. 14 Table 1. Common sphinx moths of Colorado .......................... 14 Douglas-fir Tussock Moth ....................................... 15 1. Whitney Cranshaw, Colorado State University Cooperative Extension etnomologist and associate professor, entomology; David Leatherman, entomologist, Colorado State Forest Service; Boris Kondratieff, associate professor, entomology. 8/93. ©Colorado State University Cooperative Extension. 1994. For more information, contact your county Cooperative Extension office. Issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, -

Green Fruitworms
NEW YORK'S FOOD AND LIFE SCIENCES BULLETIN NO. 50, OCTOBER 1974 NEW YORK STATE AGRICULTURAL EXPERIMENT STATION, GENEVA, A DIVISION OF THE NEW YORK STATE COLLEGE OF AGRICULTURE AND LIFE SCIENCES, A STATUTORY COLLEGE OF THE STATE UNIVERSITY, CORNELL UNIVERSITY, ITHACA Green Fruitworms P. J. Chapman and S. E. Lienk INTRODUCTION Young apple and pear fruits may be fed upon by several species of relatively large, stout-bodied green caterpillars (Fig. 1). Their dominant green color is relieved by dots, dashes, lines, and stripes of white, cream, or yellow. For more than a century now, these native insects have been known to commercial and amateur fruit growers as "green fruitworms" (6, 10, 17, 21, 22). Ten species of green fruitworms occur in New York. Tax- onomically, these constitute an artificial assemblage for while all are members of the same family (Noctuidae), four genera are represented in the group. However, six are members of the genus Lithophane. J ustif ication for treating these species as a unit rests on the fact that they form a quite distinctive pest complex. Thus, in the larval or cater- pillar stage, they are of very similar appearance and habits, feed at the same season, cause the same kind of feeding injury, and produce single generations annually. So, while the primary reason for treating these insects collectively has an economic basis, we expect the informa- tion given here will prove useful both to those having a Figure 2. —Young apple fruits showing green fruitworm technical interest in these species as well as to those hav- feeding injury. -

Zootaxa, Two New Species of the Subfamily Xyleninae from China
Zootaxa 1993: 53–60 (2009) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2009 · Magnolia Press ISSN 1175-5334 (online edition) Two new species of the subfamily Xyleninae from China (Lepidoptera, Noctuidae) VLADIMIR KONONENKO Laboratory of Entomology, Institute of Biology and Soil science Far Eastern Branch of Russian Academy of Sciences, RF-690022 Vladivostok, Russia. E-mail: [email protected] Abstract Two new noctuids in the subfamily Xyleninae, Chasminodes behouneki sp. n. and Lithophane gansuana sp. n., belonging to the tribes Cosmiini and Xylenini respectively, are described from central China. The new species C. behouneki and L. gansuana represent allopatric sister species of C. ussurica Kononenko, 1982 and L. pacifica Kononenko, 1978, both described from the Russian Far East. Adults and the genitalia of the species are illustrated, and new distributional data and maps for C. ussurica and L. pacifica are presented. A checklist of the genus Chasminodes is provided. Key words: Lepidoptera, Noctuidae, Xyleninae, Cosmiini, Xylenini, Chasminodes, Lithophane, new species, China, Russian Far East Introduction The present article two pairs of sister species of Xyleninae from Central China and the Russian Far East. These belong to two genera of two rather remote groups, the tribes Cosmiini and Xylenini in the subfamily Xyleninae (sensu Fibiger & Lafontaine 2005 and Lafontaine & Fibiger 2006). The new species represent allopatric sister species to C. ussurica and L. pacifica respectively, described from the south of Russian Far East, and found also in the Korean Peninsula and North China. Specimens used for the description of the new species have come from the private collections of German and Russian lepidopterists. -

Article: Presidential Address 1997: a New Species of Lithophane
J OURNAL OF THE LEPIDOPTERISTS' Soc1ETY Volume 52 1998 Number 1 Jou:rnalof the Lepidopterists� Society 52(1), 1998, 1-8 PRESIDENTIAL ADDRESS 1997: A NEW SPECIES OF LITHOPHANE (NOCTUIDAE), FROM THE MIDWESTERN UNITED STATES, DEDICATED TO THE PURPOSE OF THE LEPIDOPTERISTS' SOCIETY ERIC H. METZLER 1241 Kildale Square North, Columhus, Ohio 43229, USA ABSTRACT. Lithophane franclemonti, new species, is described and illustrated from Killdeer Plains Wildlife Area, Wyandot County, Ohio. The new species is most simi lar to L. innominata (Smith) and L. bethunei (Grote & Robinson). The holotype and male and female genitalia are illustrated. The description is written in deference to the princi ple of collaboration between amateur and professional lepidopterists. Additional key words: Ohio, Wisconsin, Illinois, Pennsylvania, Killdeer Plains Wildlife Area. This presidential address coincides with the 50th anniversary of The Lepidopterists' Society. At this quintessential occasion, it seems appro priate to highlight the celebration, and thus I take the liberty of straying from the traditional erudite wisdom of former Presidents by dedicating this paper to the purpose of the Society, as eloquently penned by Cyril Franklin dos Passos (Kendall 1977), to wit: "It shall be the purpose of the Society to promote internationally the science of lepi dopterology in all its branches; to further the scientifically sound and progressive study of Lepidoptera; to publish periodicals and other publications on Lepidoptera; to facilitate the exchange of speci1nens and ideas by both the professional worker and the amateur in the field; to compile and distribute information to other organizations and individuals for purposes of education and conservation and appreciation of Lep idoptera; and to secure cooperation in all measures tending to that end." The foresight and strength of this passage, now 50 years old, is sup ported by the success of the Society, and its impact on the thousands of professional and amateur enthusiasts who exemplify its meaning. -

Glenn Oakes Bioblitz Report
Glen Oakes BioBlitz Report Note: The black line in the map denotes Glen Oakes Town Forest in Fremont, N.H. From May, 2011 Glenn Oakes Bioblitz 2011 page 2 of 11 whk Introduction On and around the weekend of May 21, 2011, an intensive species inventory (BioBlitz) was made in Fremont, NH within the Glen Oakes Town Forest. The event was organized to enhance the baseline information of the biodiversity found in this area begun by Forester Charles A. Moreno. Three small teams of volunteers lead by experts spent the morning of Saturday, May 21, 2011 scouring the Glen Oakes Town Forest in three different directions keeping an inventory of the species they identified. In addition, other volunteers unable to attend the event that day reported their findings from the week before and after the event. The result of these efforts is the species inventory list found in the right-hand column (verified 5/2011) of this report. The third column from the left contains observations made by Charles A. Moreno during the period of time he was working on the Forest Management Plan for the Glen Oakes Conservation Area (2009). Together the two columns represent the observed biodiversity of this area This conservation area, a part of the larger Spruce Swamp ecosystem, provides a unique haven of biodiversity. Much of the area inventoried has been identified as “highest quality habitat” or as important “supporting habitat” in the New Hampshire Wildlife Action Plan – please see the map printed on the cover of this report. It is worth noting the area contains the critical habitats required for several Species of Greatest Conservation Concern in New Hampshire. -
Index of Scientific Names
NDEX OF CIENTIFIC AMES I S N 323 INDEX OF SCIENTIFIC NAMES A B Drepanulatrix falcataria 102 F Abagrotis duanca 176 Battus philenor 58 Drepanulatrix foeminaria 103 Feralia deceptiva 207 Abagrotis glenni 177 Biston betularia 93 Drepanulatrix monicaria 104 Feralia februalis 208 Achytonix epipaschia 178 Drepanulatrix unicalcararia 105 Fishia evelina 209 Acronicta cyanescens 179 C Dysstroma citrata 106 Furcula cinerea 249 Acronicta funeralis 180 Dysstroma formosa 107 Furcula scolopendrina 250 Campaea perlata 94 Dysstroma sobria 108 Acronicta grisea 181 Catocala aholibah 196 Acronicta hesperida 182 Catocala briseis 197 G Acronicta impleta 183 Catocala ilia 198 E Gabriola dyari 126 Acronicta impressa 184 Catocala verrilliana 199 Ectropis crepuscularia 109 Gnophaela latipennis 74 Acronicta marmorata 185 Celastrina argiolus 32 Egira crucialis 203 Grammia ornata 75 Acronicta perdita 186 Ceranemota fasciata 264 Egira curialis 204 Adelpha bredowii 44 Cercyonis pegala 69 Egira februalis 205 H Aethaloida packardaria 88 Egira perlubens 206 Chesiadodes cinerea 95 Habrodais grunus 33 Alypia langtoni 187 Chlorochlamys triangularis 96 Elpiste lorquinaria 110 Amphipyra pyramidoides 188 Ennomos magnaria 111 Habrosyne scripta 265 Chlorosea banksaria 97 Hemihyalea edwardsii 76 Anacamptodes clivinaria 89 Cisseps fulvicollis 71 Epargyreus clarus 28 Anagoga occiduaria 90 Erannis tiliaria 112 Hemileuca eglanterina 257 Cissusa indiscreta 200 Hesperumia latipennis 127 Andropolia aedon 189 Clemensia albata 72 Erynnis propertius 29 Andropolia diversilineata 190 Euchlaena -
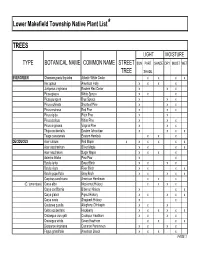
Native Plant List Trees.XLS
Lower Makefield Township Native Plant List* TREES LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE EVERGREEN Chamaecyparis thyoides Atlantic White Cedar x x x x IIex opaca American Holly x x x x Juniperus virginiana Eastern Red Cedar x x x Picea glauca White Spruce x x x Picea pungens Blue Spruce x x x Pinus echinata Shortleaf Pine x x x Pinus resinosa Red Pine x x x Pinus rigida Pitch Pine x x Pinus strobus White Pine x x x Pinus virginiana Virginia Pine x x x Thuja occidentalis Eastern Arborvitae x x x x Tsuga canadensis Eastern Hemlock xx x DECIDUOUS Acer rubrum Red Maple x x x x x x Acer saccharinum Silver Maple x x x x Acer saccharum Sugar Maple x x x x Asimina triloba Paw-Paw x x Betula lenta Sweet Birch x x x x Betula nigra River Birch x x x x Betula populifolia Gray Birch x x x x x Carpinus caroliniana American Hornbeam x x x (C. tomentosa) Carya alba Mockernut Hickory x x x x Carya cordiformis Bitternut Hickory x x x Carya glabra Pignut Hickory x x x x x Carya ovata Shagbark Hickory x x Castanea pumila Allegheny Chinkapin xx x Celtis occidentalis Hackberry x x x x x x Crataegus crus-galli Cockspur Hawthorn x x x x Crataegus viridis Green Hawthorn x x x x Diospyros virginiana Common Persimmon x x x x Fagus grandifolia American Beech x x x x PAGE 1 Exhibit 1 TREES (cont'd) LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE DECIDUOUS (cont'd) Fraxinus americana White Ash x x x x Fraxinus pennsylvanica Green Ash x x x x x Gleditsia triacanthos v.