Polymnia Canadensis L
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Prospects for Biological Control of Ambrosia Artemisiifolia in Europe: Learning from the Past
DOI: 10.1111/j.1365-3180.2011.00879.x Prospects for biological control of Ambrosia artemisiifolia in Europe: learning from the past EGERBER*,USCHAFFNER*,AGASSMANN*,HLHINZ*,MSEIER & HMU¨ LLER-SCHA¨ RERà *CABI Europe-Switzerland, Dele´mont, Switzerland, CABI Europe-UK, Egham, Surrey, UK, and àDepartment of Biology, Unit of Ecology & Evolution, University of Fribourg, Fribourg, Switzerland Received 18 November 2010 Revised version accepted 16 June 2011 Subject Editor: Paul Hatcher, Reading, UK management approach. Two fungal pathogens have Summary been reported to adversely impact A. artemisiifolia in the The recent invasion by Ambrosia artemisiifolia (common introduced range, but their biology makes them unsuit- ragweed) has, like no other plant, raised the awareness able for mass production and application as a myco- of invasive plants in Europe. The main concerns herbicide. In the native range of A. artemisiifolia, on the regarding this plant are that it produces a large amount other hand, a number of herbivores and pathogens of highly allergenic pollen that causes high rates of associated with this plant have a very narrow host range sensitisation among humans, but also A. artemisiifolia is and reduce pollen and seed production, the stage most increasingly becoming a major weed in agriculture. sensitive for long-term population management of this Recently, chemical and mechanical control methods winter annual. We discuss and propose a prioritisation have been developed and partially implemented in of these biological control candidates for a classical or Europe, but sustainable control strategies to mitigate inundative biological control approach against its spread into areas not yet invaded and to reduce its A. -

Barcoding the Asteraceae of Tennessee, Tribe Coreopsideae
Schilling, E.E., N. Mattson, and A. Floden. 2014. Barcoding the Asteraceae of Tennessee, tribe Coreopsideae. Phytoneuron 2014-101: 1–6. Published 20 October 2014. ISSN 2153 733X BARCODING THE ASTERACEAE OF TENNESSEE, TRIBE COREOPSIDEAE EDWARD E. SCHILLING, NICHOLAS MATTSON, AARON FLODEN Herbarium TENN Department of Ecology & Evolutionary Biology University of Tennessee Knoxville, Tennessee 37996 [email protected]; [email protected] ABSTRACT Results from barcoding studies of tribe Coreopsideae for the Tennessee flora using the nuclear ribosomal ITS marker are presented and include the first complete reports for 2 of the 20 species of the tribe that occur in the state, as well as updated reports for several others. Sequence data from the ITS region separate most of the species of Bidens in Tennessee from one another, but species of Coreopsis, especially those of sect. Coreopsis, have ITS sequences that are identical (or nearly so) to at least one congener. Comparisons of sequence data to GenBank records are complicated by apparent inaccuracies of older sequences as well as potentially misidentified samples. Broad survey of C. lanceolata from across its range showed little variability, but the ITS sequence of a morphologically distinct sample from a Florida limestone glade area was distinct in lacking a length polymorphism that was present in other samples. Tribe Coreopsideae is part of the Heliantheae alliance and earlier was often included in an expanded Heliantheae (Anderberg et al. 2007) in which it was usually treated as a subtribe (Crawford et al. 2009). The tribe shows a small burst of diversity in the southeastern USA involving Bidens and Coreopsis sect. -

Coreopsideae Daniel J
Chapter42 Coreopsideae Daniel J. Crawford, Mes! n Tadesse, Mark E. Mort, "ebecca T. Kimball and Christopher P. "andle HISTORICAL OVERVIEW AND PHYLOGENY In a cladistic analysis of morphological features of Heliantheae by Karis (1993), Coreopsidinae were reported Morphological data to be an ingroup within Heliantheae s.l. The group was A synthesis and analysis of the systematic information on represented in the analysis by Isostigma, Chrysanthellum, tribe Heliantheae was provided by Stuessy (1977a) with Cosmos, and Coreopsis. In a subsequent paper (Karis and indications of “three main evolutionary lines” within "yding 1994), the treatment of Coreopsidinae was the the tribe. He recognized ! fteen subtribes and, of these, same as the one provided above except for the follow- Coreopsidinae along with Fitchiinae, are considered ing: Diodontium, which was placed in synonymy with as constituting the third and smallest natural grouping Glossocardia by "obinson (1981), was reinstated following within the tribe. Coreopsidinae, including 31 genera, the work of Veldkamp and Kre# er (1991), who also rele- were divided into seven informal groups. Turner and gated Glossogyne and Guerreroia as synonyms of Glossocardia, Powell (1977), in the same work, proposed the new tribe but raised Glossogyne sect. Trionicinia to generic rank; Coreopsideae Turner & Powell but did not describe it. Eryngiophyllum was placed as a synonym of Chrysanthellum Their basis for the new tribe appears to be ! nding a suit- following the work of Turner (1988); Fitchia, which was able place for subtribe Jaumeinae. They suggested that the placed in Fitchiinae by "obinson (1981), was returned previously recognized genera of Jaumeinae ( Jaumea and to Coreopsidinae; Guardiola was left as an unassigned Venegasia) could be related to Coreopsidinae or to some Heliantheae; Guizotia and Staurochlamys were placed in members of Senecioneae. -

Download Download
IN MEMORIAM: DONALD J. PINKAVA (29 AUGUST 1933–25 JULY 2017) Liz Makings Herbarium (ASU), School of Life Sciences Arizona State University, P.O. Box 874108 Tempe, Arizona 85287-4108, U.S.A. [email protected] My name is Liz Makings and I am the collections manager of the Arizona State University Herbarium. I was a graduate student at ASU in 2000 when I met Dr. Pinkava and he had just retired, so while I missed out on his talents as a teacher, I was lucky to get to know him as a mentor, colleague, and friend. Dr. Pinkava had a heart of gold, a mind like a trap, and a delightful collection of idiosyncrasies that was perfectly suited to his career path. He was hired at ASU in 1964 after completing his PhD. at Ohio State and was immediately responsible for teaching a 300 level botany class called “Flora of Arizona.” He undertook this responsibility with a meticulousness and attention to detail that can only be described as “Pinkavesque,” col- lecting the plants, learning the flora, and scouring the state for the best field trip sites. To his students he was simultaneously feared and adored. His exams turned men into boys and triggered anxiety attacks even among the best. He did not give grades, you earned them. There was no one more demanding, no one more thorough, yet no one more caring and helpful. Many former students have sung his praises and I’ll share this quote from one: “Dr. Pinkava was one of the kindest scientists I have ever interacted with, a trait that sometimes goes missing in our academic world. -

Book of Abstracts.Pdf
1 List of presenters A A., Hudson 329 Anil Kumar, Nadesa 189 Panicker A., Kingman 329 Arnautova, Elena 150 Abeli, Thomas 168 Aronson, James 197, 326 Abu Taleb, Tariq 215 ARSLA N, Kadir 363 351Abunnasr, 288 Arvanitis, Pantelis 114 Yaser Agnello, Gaia 268 Aspetakis, Ioannis 114 Aguilar, Rudy 105 Astafieff, Katia 80, 207 Ait Babahmad, 351 Avancini, Ricardo 320 Rachid Al Issaey , 235 Awas, Tesfaye 354, 176 Ghudaina Albrecht , Matthew 326 Ay, Nurhan 78 Allan, Eric 222 Aydınkal, Rasim 31 Murat Allenstein, Pamela 38 Ayenew, Ashenafi 337 Amat De León 233 Azevedo, Carine 204 Arce, Elena An, Miao 286 B B., Von Arx 365 Bétrisey, Sébastien 113 Bang, Miin 160 Birkinshaw, Chris 326 Barblishvili, Tinatin 336 Bizard, Léa 168 Barham, Ellie 179 Bjureke, Kristina 186 Barker, Katharine 220 Blackmore, 325 Stephen Barreiro, Graciela 287 Blanchflower, Paul 94 Barreiro, Graciela 139 Boillat, Cyril 119, 279 Barteau, Benjamin 131 Bonnet, François 67 Bar-Yoseph, Adi 230 Boom, Brian 262, 141 Bauters, Kenneth 118 Boratyński, Adam 113 Bavcon, Jože 111, 110 Bouman, Roderick 15 Beck, Sarah 217 Bouteleau, Serge 287, 139 Beech, Emily 128 Bray, Laurent 350 Beech, Emily 135 Breman, Elinor 168, 170, 280 Bellefroid, Elke 166, 118, 165 Brockington, 342 Samuel Bellet Serrano, 233, 259 Brockington, 341 María Samuel Berg, Christian 168 Burkart, Michael 81 6th Global Botanic Gardens Congress, 26-30 June 2017, Geneva, Switzerland 2 C C., Sousa 329 Chen, Xiaoya 261 Cable, Stuart 312 Cheng, Hyo Cheng 160 Cabral-Oliveira, 204 Cho, YC 49 Joana Callicrate, Taylor 105 Choi, Go Eun 202 Calonje, Michael 105 Christe, Camille 113 Cao, Zhikun 270 Clark, John 105, 251 Carta, Angelino 170 Coddington, 220 Carta Jonathan Caruso, Emily 351 Cole, Chris 24 Casimiro, Pedro 244 Cook, Alexandra 212 Casino, Ana 276, 277, 318 Coombes, Allen 147 Castro, Sílvia 204 Corlett, Richard 86 Catoni, Rosangela 335 Corona Callejas , 274 Norma Edith Cavender, Nicole 84, 139 Correia, Filipe 204 Ceron Carpio , 274 Costa, João 244 Amparo B. -

Chromosome Numbers in Compositae, XII: Heliantheae
SMITHSONIAN CONTRIBUTIONS TO BOTANY 0 NCTMBER 52 Chromosome Numbers in Compositae, XII: Heliantheae Harold Robinson, A. Michael Powell, Robert M. King, andJames F. Weedin SMITHSONIAN INSTITUTION PRESS City of Washington 1981 ABSTRACT Robinson, Harold, A. Michael Powell, Robert M. King, and James F. Weedin. Chromosome Numbers in Compositae, XII: Heliantheae. Smithsonian Contri- butions to Botany, number 52, 28 pages, 3 tables, 1981.-Chromosome reports are provided for 145 populations, including first reports for 33 species and three genera, Garcilassa, Riencourtia, and Helianthopsis. Chromosome numbers are arranged according to Robinson’s recently broadened concept of the Heliantheae, with citations for 212 of the ca. 265 genera and 32 of the 35 subtribes. Diverse elements, including the Ambrosieae, typical Heliantheae, most Helenieae, the Tegeteae, and genera such as Arnica from the Senecioneae, are seen to share a specialized cytological history involving polyploid ancestry. The authors disagree with one another regarding the point at which such polyploidy occurred and on whether subtribes lacking higher numbers, such as the Galinsoginae, share the polyploid ancestry. Numerous examples of aneuploid decrease, secondary polyploidy, and some secondary aneuploid decreases are cited. The Marshalliinae are considered remote from other subtribes and close to the Inuleae. Evidence from related tribes favors an ultimate base of X = 10 for the Heliantheae and at least the subfamily As teroideae. OFFICIALPUBLICATION DATE is handstamped in a limited number of initial copies and is recorded in the Institution’s annual report, Smithsonian Year. SERIESCOVER DESIGN: Leaf clearing from the katsura tree Cercidiphyllumjaponicum Siebold and Zuccarini. Library of Congress Cataloging in Publication Data Main entry under title: Chromosome numbers in Compositae, XII. -

Heliantheae of Iowa. I
Proceedings of the Iowa Academy of Science Volume 36 Annual Issue Article 27 1929 Heliantheae of Iowa. I M. Rae Johns Let us know how access to this document benefits ouy Copyright ©1929 Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Johns, M. Rae (1929) "Heliantheae of Iowa. I," Proceedings of the Iowa Academy of Science, 36(1), 147-184. Available at: https://scholarworks.uni.edu/pias/vol36/iss1/27 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Johns: Heliantheae of Iowa. I HELIANTHEAE OF row A. I lVI. RAE JOIINS Heliantheae is one of the nine tribes of the Family Compositae. It is believed to have originated in South America soon after the appearance of the Family and may be regarded as the oldest and most primitive of the tribes. The near approach to the earliest composite form is said to be retained in the more foliaceous outer invol ucral bracts, the more normally developed and more firmly attached paleae subtending the disk flowers, the 0 less transformed calyx-limb of persistent teeth or aristae directly continuous with the ribs of the ovary, and in some genera, the less firmly united or nearly free anthers. (Bentham, 1873.) The characters which today distinguish it from the other tribes are: Receptacle: chaffy, depressed, horizontal, convex, or columnar. -

Illinois Bundleflower (Desmanthus Illinoensis) Story by Alan Shadow, Manager USDA-NRCS East Texas Plant Materials Center Nacogdoches, Texas
Helping People Help The Land September/October 2011 Issue No. 11 The Reverchon Naturalist Recognizing the work of French botanist Julien Reverchon, who began collecting throughout the North Central Texas area in 1876, and all the botanists/naturalists who have followed ... Drought, Heat and Native Trees ranging from simple things like more extensive root systems, to more drastic measures like pre- Story by Bruce Kreitler mature defoliation, what they actually have little Abilene, Texas defense against is a very prolonged period of no appreciable water supply. nybody that has traveled in Texas this year A will have noticed that not only most of the By the way, even though they are usually the land browned out, but also if you look at the trees same species, there is a difference in landscape in the fields and beside the roads, they aren't trees and native trees, which are untended plants looking so good either. It doesn't take a rocket that have to fend for themselves. While they are scientist to realize that extreme high temperatures indeed the same basic trees, the differences be- combined with, and partially caused by, drought tween the environments that they live in are huge are hard on trees. and thus overall general environmental factors such as drought, temperature, and insect infesta- Since I'm pretty sure that most of the people read- tions act on them differently. For the purposes of ing this article understand very well that drought this article, I'm referring to trees that are on their is a problem for trees, the question isn't is the pre- own, untended for their entire lives in fields, pas- sent drought going to have an effect on trees, but tures, forests, or just wherever nature has placed rather, what are the present effects of the drought them and refer to them as native trees. -

2020 Plant List Index: Trees & Shrubs Pg
2020 Plant List Index: Trees & Shrubs pg. 2-7 Perennials pg. 7-13 Grasses pg. 14 Ferns pg. 14-15 Vines pg. 15 Hours: May 1 – June 30: Tues.- Sat. 10 am - 6 pm Sun.11 am - 5 pm 3351 State Route 37 West www.sciotogardens.com On Mondays by appointment Delaware, OH 43015 Phone/fax: 740-363-8264 Email: [email protected] Sustainable, earth-friendly growth and maintenance practices: Real Soil = Real Difference. All plants are container-grown in a blend of local soil and compost. Plants are grown outside year-round. They are always in step with the seasons. Minimal pruning ensures a well-rooted, healthy plant. Use degradableRoot Pouch andcontainers. recycled containers to reduce waste. Use of controlled-release fertilizers minimizes leaching into the environment. Our primary focus is on native plants. However, non-invasive exotics are an equally important part of the choices we offer you. There is great creative opportunity using natives in combination with exotics. Adding more native plants into our landscapes provides food and habitat for wildlife and connections to larger natural areas. AdditionalAdditional species species may may be be available. available. Email Email oror call for currentcurrent availability, availability, sizes, sizes, and and prices. prices. «BOT_NAME» «BOT_NAME»Wetland Indicator Status—This is listed in parentheses after the common name when a status is known. All species «COM_NAM» «COM_NAM» «DESCRIP»have not been evaluated. The indicator code is helpful in evaluating«DESCRIP» the appropriate habitat for a -
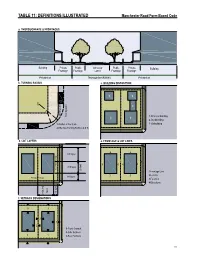
Manchester Road Redevelopment District: Form-Based Code
TaBle 11: deFiniTionS illuSTraTed manchester road Form-Based Code a. ThoroughFare & FronTageS Building Private Public Vehicular Public Private Building Frontage Frontage Lanes Frontage Frontage Private lot Thoroughfare (r.o.w.) Private lot b. Turning radiuS c. Building diSPoSiTion 3 3 2 2 1 Parking Lane Moving Lane 1- Principal Building 1 1 2- Backbuilding 1-Radius at the Curb 3- Outbuilding 2-Effective Turning Radius (± 8 ft) d. loT LAYERS e. FronTage & loT lineS 4 3rd layer 4 2 1 4 4 4 3 2nd layer Secondary Frontage 20 feet 1-Frontage Line 2-Lot Line 1st layer 3 3 Principal Frontage 3-Facades 1 1 4-Elevations layer 1st layer 2nd & 3rd & 2nd f. SeTBaCk deSignaTionS 3 3 2 1 2 1-Front Setback 2-Side Setback 1 1 3-Rear Setback 111 Manchester Road Form-Based Code ARTICLE 9. APPENDIX MATERIALS MBG Kemper Center PlantFinder About PlantFinder List of Gardens Visit Gardens Alphabetical List Common Names Search E-Mail Questions Menu Quick Links Home Page Your Plant Search Results Kemper Blog PlantFinder Please Note: The following plants all meet your search criteria. This list is not necessarily a list of recommended plants to grow, however. Please read about each PF Search Manchesterplant. Some may Road be invasive Form-Based in your area or may Code have undesirable characteristics such as above averageTab insect LEor disease 11: problems. NATIVE PLANT LIST Pests Plants of Merit Missouri Native Plant List provided by the Missouri Botanical Garden PlantFinder http://www.mobot.org/gardeninghelp/plantfinder Master Search Search limited to: Missouri Natives Search Tips Scientific Name Scientific Name Common NameCommon Name Height (ft.) ZoneZone GardeningHelp (ft.) Acer negundo box elder 30-50 2-10 Acer rubrum red maple 40-70 3-9 Acer saccharinum silver maple 50-80 3-9 Titles Acer saccharum sugar maple 40-80 3-8 Acer saccharum subsp. -

Ranunculaceae – Buttercup Family
RANUNCULACEAE – BUTTERCUP FAMILY Plant: mostly herbs, some woody vines or shrubs Stem: Root: Leaves: mostly alternate, sometimes opposite or whorled or basal; lobed or not lobed; if lobed then most often palmately, but occasionally pinnately, sometimes finely dissected – highly variable, sometimes even on the same plant; with or without stipules Flowers: mostly perfect, some dioecious; sepals 3-6, commonly 5; petals vary in number (3-23) but often 5, petals may be lacking and sepals are showy; stamens few to many; ovary superior, carpels few to very many, pistils one to many Fruit: mostly a dry capsule, seeds small, may be oily; rarely a berry Other: large family, sometimes confused with members of the Rose family (5 petals); Dicotyledons Group Genera: 60+ genera; locally Actaea (baneberry), Anemone (anemone or windflower), Aquilegia (columbine), Clematis, Isopyrum, Hepatica, Hydrastis, Ranunuculus (buttercup or crowfoot), Thalictrum (meadow-rue) WARNING – family descriptions are only a layman’s guide and should not be used as definitive Flower Morphology in the This is a large family often based on 5’s but Ranunculaceae (Buttercup Family) exceptions occur Examples of common genera White Baneberry [Doll’s-Eyes] Yellow Marsh Marigold [Cowslip] Goldenseal [Yellowroot] Actaea pachypoda Ell. Carolina [Wild Blue] Larkspur Caltha palustris L. var. palustris Delphinium carolinianum Walter Hydrastis canadensis L. Swamp Leather Flower [Eastern] False Rue Anemone Clematis crispa L. Devil-In-The-Bush [Love American Wood Anemone Enemion biternatum Raf. -In-A-Mist] Anemone quinquefolia L. [Isopyrum biternatum] Nigella damascena L. (Introduced) Doubtful [Rocket; Garden] Knight's-Spur [Larkspur] Round-lobed Hepatica [Liverleaf] Tall Buttercup Hepatica nobilis Schreber var. -

Plant Introductions
Plant Introductions Promoting the garden use of native plants is a core part of Mt. Cuba Center’s mission, and since 1988, the Plant Introduction Program has brought many outstanding cultivars to market. Selected for strong ornamental appeal and broad regional adaptability, many of these introductions have risen to become some of the most popular cultivars of native plants available. Plant evaluation and introduction continues today, with several bright prospects for additional introductions in the future. Plant Introductions Actaea pachypoda ‘Misty Blue’ Misty Blue white baneberry Misty Blue white baneberry is an herbaceous perennial, selected for its unique and highly attractive soft, bluish-green foliage. It was discovered in a planting of typical green-leaved plants of unknown origin growing at Mt. Cuba Center. In spring 1" to 2" tall bottlebrush-like clusters of white flowers are borne on stems above the foliage. By September, large, white fruit with dark purple to black spots (“doll’s eyes”) mature on reddish pedicels. It is a carefree, long-lived, 24" to 36" tall plant, growing best in partial to filtered shade in evenly moist, well-drained soils with a pH from slightly acidic to neutral. Introduced 2009 Ageratina altissima ‘Chocolate’ (formerly Eupatorium rugosum) Chocolate white snakeroot Chocolate white snakeroot is an herbaceous perennial selected by Dr. Richard W. Lighty and descended from a plant found at Winterthur Gardens. It had the darkest burgundy foliage of many seedlings grown over a ten year period. ‘Chocolate’ grows up to 3' tall and has white inflorescences along with dark burgundy leaves which color best in full sun.