Read Book Elements
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

The Past and Future of the Periodic Table
The Past and Future of the Periodic Table This stalwart symbol of the field of chemistry always faces scrutiny and debate Eric R. Scerri t graces the walls of lecture halls making up one period. The lengths elements, Döbereiner found that the Iand laboratories of all types, from of periods vary: The first has two ele- weight of the middle element—such as universities to industry. It is one of ments, the next two eight each, then 18 selenium in the triad formed by sulfur, the most powerful icons of science. It and 32, respectively, for the next pairs selenium and tellurium—had an atom- captures the essence of chemistry in of periods. Vertical columns make up ic weight that was the approximate one elegant pattern. The periodic table groups, of which there are 18, based average of the weights of the other two provides a concise way of understand- on similar chemical properties, related elements. Sulfur’s atomic weight, in ing how all known chemical elements to the number of electrons in the outer Döbereiner’s time, was 32.239, where- react with one another and enter into shell of the atoms, also called the va- as tellurium’s was 129.243, the average chemical bonding, and it helps to ex- lence shell. For instance, group 17, the of which is 80.741, or close to the then- plain the properties of each element halogens, all lack one electron to fill measured value for selenium, 79.264. that make it react in such a fashion. their valence shells, all tend to acquire The importance of this discovery lay But the periodic system is so funda- electrons during reactions, and all form in the marrying of qualitative chemical mental, pervasive and familiar in the acids with hydrogen. -

POST MENDELEEVIAN EVOLUTION of the PERIODIC TABLE (.Pdf)
Post Mendeleevian Evolution of the Periodic Table Gary Katz Periodic Round Table We are here in the centenary of the death of Mendeleev, whose life defines the epoch of the Periodic Table. To his memory we respectfully dedicate this, our plan to revitalize his invention, the Periodic Table. Since Mendeleev’s death in 1907, the only substantive changes in the Table have been the insertion of new elements; most fall into the eighth period with another seven in the main body of the Table, but only three of these are stable non-radioactive species. The quantum chemical results of Niels Bohr came along early in the century elucidating the electronic con- figurations of the atoms, explaining a great deal about what made the Periodic Table periodic. By mid century when Seaborg proposed an actinide series to parallel the lanthanide series, including a number of the new transuranium elements, the official Table we now have was es- sentially in place. It has not changed fundamentally since that time. In my previous paper “An Eight-period Table for the 21st Century” (1) I discussed the evolution of the 8-period concept and some of the people who have worked toward the goal of transforming the Periodic Table in this fashion. Since 2001, I have changed my terminology to agree with that of other writers: the 8-period table is now to be called the left-step table. This is a phrase coined by physical chemist Henry Bent, who proposed it back in the 80’s, and it allows for the expansion of the table into periods beyond the eighth—a critical area of future development for the Periodic Table. -

Upper Limit in Mendeleev's Periodic Table Element No.155
AMERICAN RESEARCH PRESS Upper Limit in Mendeleev’s Periodic Table Element No.155 by Albert Khazan Third Edition — 2012 American Research Press Albert Khazan Upper Limit in Mendeleev’s Periodic Table — Element No. 155 Third Edition with some recent amendments contained in new chapters Edited and prefaced by Dmitri Rabounski Editor-in-Chief of Progress in Physics and The Abraham Zelmanov Journal Rehoboth, New Mexico, USA — 2012 — This book can be ordered in a paper bound reprint from: Books on Demand, ProQuest Information and Learning (University of Microfilm International) 300 N. Zeeb Road, P. O. Box 1346, Ann Arbor, MI 48106-1346, USA Tel.: 1-800-521-0600 (Customer Service) http://wwwlib.umi.com/bod/ This book can be ordered on-line from: Publishing Online, Co. (Seattle, Washington State) http://PublishingOnline.com Copyright c Albert Khazan, 2009, 2010, 2012 All rights reserved. Electronic copying, print copying and distribution of this book for non-commercial, academic or individual use can be made by any user without permission or charge. Any part of this book being cited or used howsoever in other publications must acknowledge this publication. No part of this book may be re- produced in any form whatsoever (including storage in any media) for commercial use without the prior permission of the copyright holder. Requests for permission to reproduce any part of this book for commercial use must be addressed to the Author. The Author retains his rights to use this book as a whole or any part of it in any other publications and in any way he sees fit. -

Electron Configuration from Wikipedia, the Free Encyclopedia
Electron configuration From Wikipedia, the free encyclopedia (Redirected from Electronic configuration) Jump to: navigation, search Electron atomic and molecular orbitals A simple electron shell diagram of lithium-7 In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals.[1] For example, the electron configuration of the neon atom is 1s2 2s2 2p6. According to the laws of quantum mechanics, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one orbital to another by emission or absorption of a quantum of energy, in the form of a photon. Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials this same idea helps explain the peculiar properties of lasers and semiconductors. Contents [hide] • 1 Shells and subshells • 2 Notation • 3 Energy — ground state and excited states • 4 History • 5 Aufbau principle and Madelung rule o 5.1 Periodic table o 5.2 Shortcomings of the Aufbau principle o 5.3 Ionization of the transition metals o 5.4 Other exceptions to Madelung's rule • 6 Electron configuration in molecules o 6.1 Electron configuration in solids • 7 Applications • 8 See also • 9 Notes • 10 References • 11 External links [edit] Shells and subshells See also: Electron shell s (l=0) p (l=1) m=0 m=0 m=±1 s pz px py n=1 n=2 Electron configuration was first conceived of under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons. -

Tetrahedral and Spherical Representations of the Periodic System
Found Chem DOI 10.1007/s10698-017-9299-y Tetrahedral and spherical representations of the periodic system Philip J. Stewart1 Ó The Author(s) 2017. This article is an open access publication Abstract The s, p, d and f blocks of the elements, as delimited by Charles Janet in 1928, can be represented as parallel slices of a regular tetrahedron. They also fit neatly on to the surface of a sphere. The reasons for this are discussed and the possible objections examined. An attempt is made to see whether there are philosophical implications of this unexpected geometrical regularity. A new tetrahedral design in transparent plastic is presented. Keywords Periodic system Á Electronic structure Á Status of helium Plato, in his dialogue Timaeus, supposed that the atoms of the four elements were in the form of four of the regular polyhedra, while the fifth—the dodecahedron—‘was used in the delineation of the inhabited world’. After the initial shock of learning that there are at least 120 existing or possible elements, he might have been pleased to know that they can be represented as four parallel and equally spaced slices of a regular tetrahedron. This has been demonstrated by Valery Tsimmerman, an American engineer, Fig. 1. The tetrahedral symmetry had also been noticed by Jess Tauber in 1979 (personal communication) and 2000. Tsimmerman’s starting point was the blocks of chemical elements as delimited by Janet (1928), a French engineer and amateur biologist and geologist, who turned his mind to the Periodic System at the age of 78. Janet arrived at his ‘left-step table’ by moving what is now called the s block from the left-hand side of the long form of the table to the right- hand side, placing H and He at its head and making Li and Be into the second period. -

Peaceful Berkelium
in your element Peaceful berkelium The first new element produced after the Second World War has led a rather peaceful life since entering the period table — until it became the target of those producing superheavy elements, as Andreas Trabesinger describes. f nomenclature were transitive, element 97 with the finding that in Bk(iii) compounds would be named after an Irish bishop spin–orbit coupling leads to a mixing of the Iwhose philosophical belief was that first excited state and the ground state. This material things do not exist. But between gives rise to unexpected electronic properties the immaterialist George Berkeley and the not present in analogous lanthanide element berkelium stands, of course, the structures containing terbium. Even more fine Californian city of Berkeley (pictured), recently5, a combined experimental and where the element was first produced in computational study revealed that berkelium December 1949. The city became the eponym can have stabilized +iii and +iV oxidation of element 97 “in a manner similar to that states also under mild aqueous conditions, used in naming its chemical homologue indicating a path to separating it from other terbium […] whose name was derived from lanthanides and actinides. the town of Ytterby, Sweden, where the rare The main use of berkelium, however — earth minerals were first found”1. arguably one that wouldn’t have met with A great honour for the city? The mayor Bishop Berkeley’s approval — remains a of Berkeley at the time reportedly displayed PHOTO STOCK / ALAMY AF ARCHIVE distinctly material one: as the target for “a complete lack of interest when he was the synthesis of other transuranium and called with the glad tidings”2. -

The Chemical Complexities of Plutonium David L
The Chemical Complexities of Plutonium David L. Clark ew people have ever seen plutonium, and far fewer have actually handled or manipu- lated it. Yet this manmade element has arguably altered the course of civilization as Fmuch as copper, bronze, iron, or steel. Within five years of its synthesis, the primary use of plutonium was for the release of nuclear energy in weapons of mass destruction, and it seemed that the new element might lead the human race to the brink of self-annihilation. But instead, plutonium has become a stabilizing agent in global politics, forcing the human race to govern itself without resorting to nuclear war. Never before has a simple chemical element had such a profound impact on the consciousness of mankind. Plutonium has had a similarly humbling impact in the more circumscribed arena of science. Incredibly, it displays physicochemical behaviors that are among the most complex of any element in the periodic table. The pure element exhibits seven distinct crystal phases, is highly reactive, and is known to form compounds, complexes, or alloys with virtually every other element. Molten plutonium is highly corrosive and will slowly react with its container, causing difficulties for handling. When elemental plutonium reacts to give up its valence electrons, it can form a wide variety of positively charged ions with the ability to form up to twelve chemical bonds to other ions or molecules in solution. The element can exhibit five oxidation states, and under certain chemical conditions, four different oxidation states can be present in appreciable amounts simultaneously! No other element displays such a complex chemistry. -
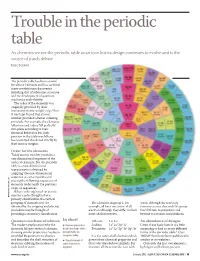
Trouble in the Periodic Table
Trouble in the periodic table As chemists we see the periodic table as an icon but its design continues to evolve and is the source of much debate Eric Scerri The periodic table has been around for almost 140 years and has survived many revolutionary discoveries including that of subatomic structure and the development of quantum mechanics and relativity. The order of the elements was originally provided by their increasing atomic weights (fig 1) but it was later found that atomic number provided a better ordering principle. For example, the elements tellurium and iodine fell perfectly into place according to their chemical behaviour but their position in the table would have been inverted if ordered strictly by their atomic weights. Order for the elements Today atomic number provides a one-dimensional sequence of the ordered elements. But the periodic table is a two-dimensional representation obtained by ‘snipping’ the one-dimensional sequence at certain points and placing the following sequences of elements underneath the previous strips or sequences. Where ordering based on atomic WWW.MAYANPERIODIC.COM number can be thought of as a primary classification, the vertical grouping of elements that are The elements in group 1, for water, although the reactivity obtained by the snipping and placing example, all have one outer-shell increases as one descends the group procedure may be thought of electron although they differ in their from lithium to potassium and providing a secondary classification. inner shell structures. beyond to caesium -

History of the Seaborg Institute
HISTORY OF THE SEABORG INSTITUTE ARQ.2_09.cover.indd 1 6/22/09 9:24 AM ContentsContents History of the Seaborg Institute The Seaborg Institute for Transactinium Science Established to educate the next generation of nuclear scientists 1 ITS Advisory Council 5 Seaborg the scientist 7 Naming seaborgium 11 Branching out 13 IGCAR honors Seaborg’s contributions to actinide science 16 Seaborg Institute for Transactinium Science/Los Alamos National Laboratory Actinide Research Quarterly The Seaborg Institute for Transactinium Science Established to educate the next generation of nuclear scientists In the late 1970s and early 1980s, research opportunities in heavy element This article was contributed by science and engineering were seldom found in university settings, and the supply Darleane C. Hoffman, professor emerita, of professionals in those fields was diminishing to the detriment of national Graduate School, Department of goals. In response to these concerns, national studies were conducted and panels Chemistry, UC Berkeley, faculty senior and committees were formed to assess the status of training and education in the scientist, Lawrence Berkeley National nuclear sciences. The studies in particular addressed the future need for scientists Laboratory, and charter director, Seaborg trained in the areas of nuclear waste management and disposal, environmental Institute, Lawrence Livermore National remediation, nuclear fuel processing, and nuclear safety analysis. Laboratory; and Christopher Gatrousis, Unfortunately, with the exception of the American Chemical Society’s former associate director of Chemistry Division of Nuclear Chemistry and Technology summer schools for undergradu- and Materials Science, Lawrence ates in nuclear and radiochemistry established in 1984, the studies did not lead Livermore National Laboratory. -

A Quantitative Evaluation of the Modal Distribution Of
A QUANTITATIVE EVALUATION OF THE MODAL DISTRIBUTION OF MINERALS IN COAL DEPOSITS IN THE HIGHVELD AREA ANDTHE ASSOCIATED IMPACT ON THE GENERATION OF ACID AND NEUTRAL MINE DRAINAGE KL Pinetown • RH Boer WRC Report No. 1264/1/06 Water Research Commission A QUANTITATIVE EVALUATION OF THE MODAL DISTRIBUTION OF MINERALS IN COAL DEPOSITS IN THE HIGHVELD AREA AND THE ASSOCIATED IMPACT ON THE GENERATION OF ACID AND NEUTRAL MINE DRAINAGE Report to the Water Research Commission by KL PlNETOWN AND RH BOER on behalf of the Department of Geology University of the Free State WRC Report No. 1264/1/06 ISBN No. 1-77005-440-5 MAY 2006 Disclaimer This report emanates from a project financed by the Water Research Commission (W'RC) and is approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the W'RC or the members of the project steering committee, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. Primed tn Silowa Primers: Hi: Hii4 MM EXECUTIVE SUMMARY The objective of this investigation was to gain a qualitative understanding of the mineralogy of the coal measures occurring in the Highveld coalfield of South Africa. The project focused on the identification of minerals occurring in coal and the understanding of the distribution of these minerals among the various coal seams. Furthermore, the lateral distribution patterns of minerals in coal were researched. In addition, an effort was made to establish the relationship between the mineralogy of the coal and the associated water quality. -

Institute for Transuranium Elements Karlsruhe
INSTITUTE FOR TRANSURANIUM ELEMENTS KARLSRUHE EUR 5381e A V bri » , ^r-— fa um ÉHEf «f i > ^^^^^· w ^R 1 ^1 ^ Bui of the K a f( s rul le' Λ li c/c'J| **WMÌIIHut e /or Trans uran]Im i F.lcMãmm : Λ i - 4 THE EUROPEAN INSTITUTE FOR TRANSURANIUM ELEMENTS (EURATOM) KARLSRUHE 1975 How to get to the Institute >c, Main Geographischer Verlag Stuttgar rsbach ».^KltAl-í ; åe^JO^"J ' - aeri" 2 4 e S kn l'Airopäisdics lnsiiiiil für Transurane 75 Karlsruhe I. Poitfach 2266 Bundesrepublik Deutschland Telex euru d 07825483 Telefon 07247/841 CONTENTS Preface I. The Institute, its Background and How it Works 7 The Transuranium Elements 7 The European Communities and their Treaties 13 Some Historic Dates 14 Facts and Figures 16 The Management of the Plutonium and Transplutonium Programme 21 Safety First 24 Beyond the Day's Work... 27 The City with the European Touch 29 The European School of Karlsruhe 30 II. Some Results and Achievements 32 Fuel Science and Technology 33 Fuel Preparation and Characterisation 33 Fuel Property Studies 34 Thermodynamics 34 Thermal Properties 36 Phase Diagrams 36 Study of Lattice Defects 37 Diffusion Studies 39 Study of Fuel under Irradiation Design and Execution of Irradiation Experiments 41 Post Irradiation Structural Analysis 42 Simulation Experiments 45 Swelling of Advanced Fuels 45 Corrosion Studies 46 Modelling Work 48 Isotope Analysis 49 Basic Actinide Research 50 Isolation and Purification of Transplutonium Nuclides 50 Preparation of Actinide, Metals and Compounds 51 Chemical and Physical Properties of Actinide Metals and Alloys 52 Selected Bibliography 55 Published by the Commission of the European Communities Directorate-General Scientific and Technical Information and Information Management Luxembourg, 1975 Compiled and edited by H.E.