A Role of Ultrabithorax in Morphological Differences Between Drosophila Species
Total Page:16
File Type:pdf, Size:1020Kb
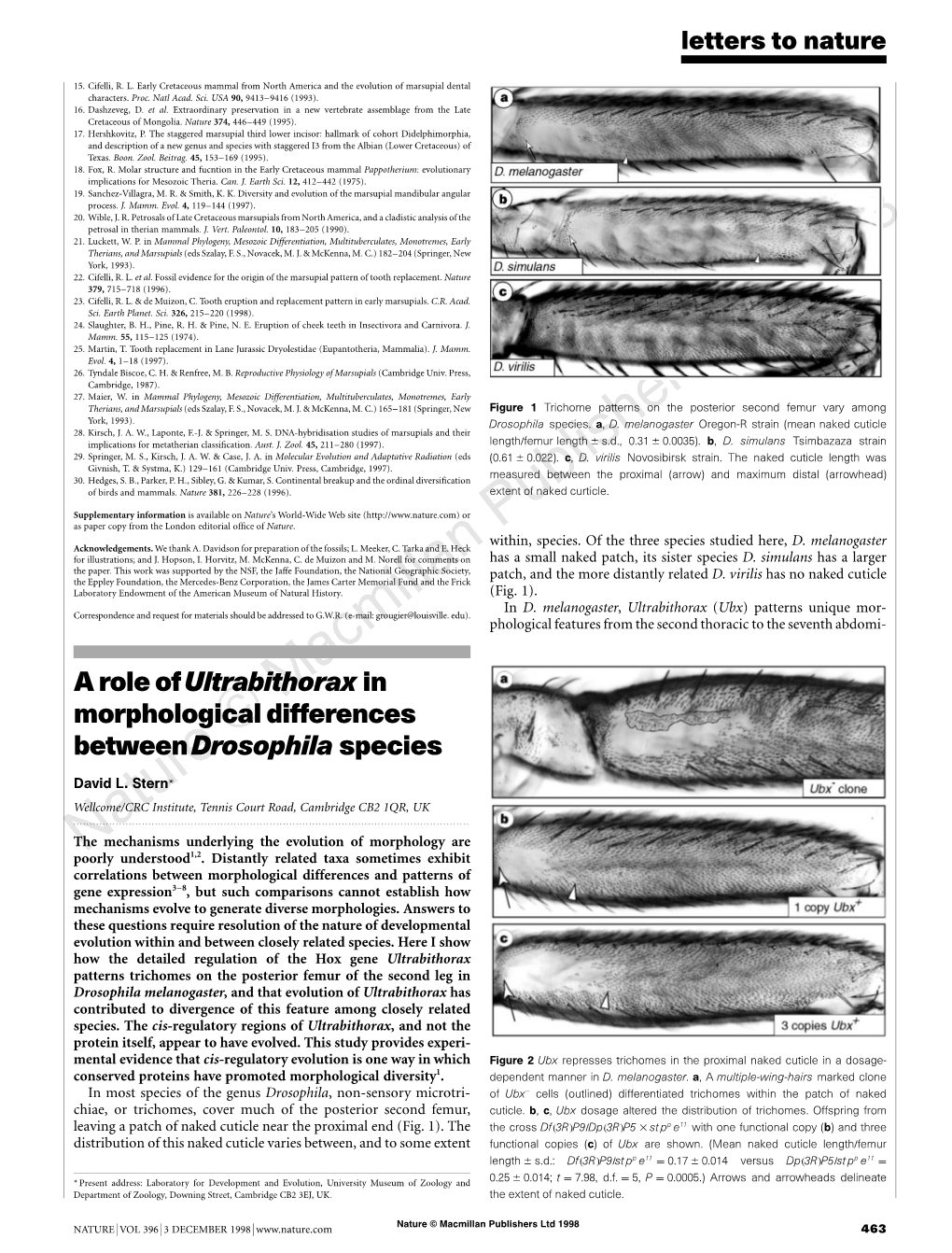
Load more
Recommended publications
-

Long Noncoding Rnas: Functional Surprises from the RNA World
Downloaded from genesdev.cshlp.org on September 25, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Long noncoding RNAs: functional surprises from the RNA world Jeremy E. Wilusz,1 Hongjae Sunwoo,2 and David L. Spector1,3 1Watson School of Biological Sciences, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 11724, USA; 2Graduate Program in Molecular and Cellular Biology, Stony Brook University, Stony Brook, New York 11794 Most of the eukaryotic genome is transcribed, yielding complexity. In addition, there has been an explosion of a complex network of transcripts that includes tens of research addressing possible functional roles for the thousands of long noncoding RNAs with little or no other 98% of the human genome that does not encode protein-coding capacity. Although the vast majority of proteins. Rather unexpectedly, transcription is not lim- long noncoding RNAs have yet to be characterized thor- ited to protein-coding regions, but is instead pervasive oughly, many of these transcripts are unlikely to represent throughout the mammalian genome as demonstrated by transcriptional ‘‘noise’’ as a significant number have been large-scale cDNA cloning projects (Carninci et al. 2005; shown to exhibit cell type-specific expression, localiza- Katayama et al. 2005) and genomic tiling arrays (Bertone tion to subcellular compartments, and association with et al. 2004; Cheng et al. 2005; Birney et al. 2007; Kapranov human diseases. Here, we highlight recent efforts that et al. 2007a). In fact, >90% of the human genome is likely have identified a myriad of molecular functions for long to be transcribed (Birney et al. 2007), yielding a complex noncoding RNAs. -

Perspectives
Copyright 0 1994 by the Genetics Society of America Perspectives Anecdotal, Historical and Critical Commentaries on Genetics Edited by James F. Crow and William F. Dove A Century of Homeosis, A Decade of Homeoboxes William McGinnis Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, Connecticut 06520-8114 NE hundred years ago, while the science of genet- ing mammals, and were proposed to encode DNA- 0 ics still existed only in the yellowing reprints of a binding homeodomainsbecause of a faint resemblance recently deceased Moravian abbot, WILLIAMBATESON to mating-type transcriptional regulatory proteins of (1894) coined the term homeosis to define a class of budding yeast and an even fainter resemblance to bac- biological variations in whichone elementof a segmen- terial helix-turn-helix transcriptional regulators. tally repeated array of organismal structures is trans- The initial stream of papers was a prelude to a flood formed toward the identity of another. After the redis- concerning homeobox genes and homeodomain pro- coveryof MENDEL’Sgenetic principles, BATESONand teins, a flood that has channeled into a steady river of others (reviewed in BATESON1909) realized that some homeo-publications, fed by many tributaries. A major examples of homeosis in floral organs and animal skel- reason for the continuing flow of studies is that many etons could be attributed to variation in genes. Soon groups, working on disparate lines of research, have thereafter, as the discipline of Drosophila genetics was found themselves swept up in the currents when they born and was evolving into a formidable intellectual found that their favorite protein contained one of the force enriching many biologicalsubjects, it gradually be- many subtypes of homeodomain. -

Phosphorylation, Expression and Function of the Ultrabithorax Protein Family in Drosophila Melanogaster
Development 112, 1077-1093 (1991) 1077 Printed in Great Britain © The Company of Biologists Limited 1991 Phosphorylation, expression and function of the Ultrabithorax protein family in Drosophila melanogaster ELIZABETH R. GAVIS* and DAVID S. HOGNESSt't Department of Biochemistry, Beckman Center, Stanford University School of Medicine, Stanford, California 94305 USA * Present address: Whitehead Institute for Biomedical Research, 9 Cambridge Center, Cambridge, Massachusetts 02142 USA t Present address: Department of Developmental Biology, Beckman Center, Stanford University School of Medicine, Stanford, California, 94305 USA $ Author for correspondence Summary Alternative splicing of the Ultrabithorax homeotic gene parallel those for the respective mRNAs, and all transcript generates a family of five proteins (UBX isoforms are similarly phosphorylated throughout em- isoforms) that function as transcription factors. All bryogenesis. Analysis by cotransfection assays of the isoforms contain a homeodomain within a common 99 aa promoter activation and repression functions of mutant C-terminal region (C-constant region) which is joined to UBX proteins with various deletions in the N-constant a common 247 aa N-terminal (N-constant) region by region shows that repression is generally insensitive to different combinations of three small optional elements. deletion and, hence, presumably to phosphorylation. By Unlike the UBX proteins expressed in E. coli, UBX contrast, the activation function is differentially sensitive isoforms expressed in D. melanogaster cells are phos- to the different deletions in a manner indicating the phorylated on serine and threonine residues, located absence of a discrete activating domain and instead, the primarily within a 53 aa region near the middle of the presence of multiple activating sequences spread N-constant region, to form at least five phosphorylated throughout the region. -

Mechanisms of Wnt Signaling in Development
P1: APR/ary P2: ARS/dat QC: ARS/APM T1: ARS August 29, 1998 9:42 Annual Reviews AR066-03 Annu. Rev. Cell Dev. Biol. 1998. 14:59–88 Copyright c 1998 by Annual Reviews. All rights reserved MECHANISMS OF WNT SIGNALING IN DEVELOPMENT Andreas Wodarz Institut f¨ur Genetik, Universit¨at D¨usseldorf, Universit¨atsstrasse 1, 40225 D¨usseldorf, Germany; e-mail: [email protected] Roel Nusse Howard Hughes Medical Institute and Department of Developmental Biology, Stanford University, Stanford, CA 94305-5428; e-mail: [email protected] KEY WORDS: Wnt, wingless, frizzled, catenin, signal transduction ABSTRACT Wnt genes encode a large family of secreted, cysteine-rich proteins that play key roles as intercellular signaling molecules in development. Genetic studies in Drosophila and Caenorhabditis elegans, ectopic gene expression in Xenopus, and gene knockouts in the mouse have demonstrated the involvement of Wnts in pro- cesses as diverse as segmentation, CNS patterning, and control of asymmetric cell divisions. The transduction of Wnt signals between cells proceeds in a complex series of events including post-translational modification and secretion of Wnts, binding to transmembrane receptors, activation of cytoplasmic effectors, and, finally, transcriptional regulation of target genes. Over the past two years our understanding of Wnt signaling has been substantially improved by the identifi- cation of Frizzled proteins as cell surface receptors for Wnts and by the finding that -catenin, a component downstream of the receptor, can translocate to the nucleus and function as a transcriptional activator. Here we review recent data that have started to unravel the mechanisms of Wnt signaling. -

Mrg15 Stimulates Ash1 H3K36 Methyltransferase Activity and Facilitates Ash1 Trithorax Group Protein Function in Drosophila
ARTICLE DOI: 10.1038/s41467-017-01897-3 OPEN Mrg15 stimulates Ash1 H3K36 methyltransferase activity and facilitates Ash1 Trithorax group protein function in Drosophila Chang Huang1, Fu Yang2, Zhuqiang Zhang1, Jing Zhang1, Gaihong Cai2, Lin Li2, Yong Zheng1, She Chen2, Rongwen Xi2 & Bing Zhu1,3 fi 1234567890 Ash1 is a Trithorax group protein that possesses H3K36-speci c histone methyltransferase activity, which antagonizes Polycomb silencing. Here we report the identification of two Ash1 complex subunits, Mrg15 and Nurf55. In vitro, Mrg15 stimulates the enzymatic activity of Ash1. In vivo, Mrg15 is recruited by Ash1 to their common targets, and Mrg15 reinforces Ash1 chromatin association and facilitates the proper deposition of H3K36me2. To dissect the functional role of Mrg15 in the context of the Ash1 complex, we identify an Ash1 point mutation (Ash1-R1288A) that displays a greatly attenuated interaction with Mrg15. Knock-in flies bearing this mutation display multiple homeotic transformation phenotypes, and these phenotypes are partially rescued by overexpressing the Mrg15-Nurf55 fusion protein, which stabilizes the association of Mrg15 with Ash1. In summary, Mrg15 is a subunit of the Ash1 complex, a stimulator of Ash1 enzymatic activity and a critical regulator of the TrxG protein function of Ash1 in Drosophila. 1 National Laboratory of Biomacromolecules, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China. 2 National institute of Biological Sciences, Beijing 102206, China. 3 College of Life Sciences, University of Chinese Academy of Sciences, Beijing 100049, China. Chang Huang, Fu Yang and Zhuqiang Zhang contributed equally to this work. Correspondence and requests for materials should be addressed to R.X. -

Control of Tissue Morphogenesis by the HOX Gene Ultrabithorax Maria-Del-Carmen Diaz-De-La-Loza1, Ryan Loker2,3, Richard S
© 2020. Published by The Company of Biologists Ltd | Development (2020) 147, dev184564. doi:10.1242/dev.184564 RESEARCH ARTICLE Control of tissue morphogenesis by the HOX gene Ultrabithorax Maria-del-Carmen Diaz-de-la-Loza1, Ryan Loker2,3, Richard S. Mann2,3 and Barry J. Thompson1,4,* ABSTRACT domain named the ‘homeobox’ that is found throughout the HOX Mutations in the Ultrabithorax (Ubx) gene cause homeotic family of transcription factors (Affolter et al., 1990a,b, 2008; Akam transformation of the normally two-winged Drosophila into a four- et al., 1984; Akam, 1983; Beachy et al., 1985; Bender et al., 1983; winged mutant fly. Ubx encodes a HOX family transcription factor that Casanova et al., 1985; Chan et al., 1994; Chan and Mann, 1993; specifies segment identity, including transformation of the second set Desplan et al., 1988; Gehring, 1992; Mann and Hogness, 1990; of wings into rudimentary halteres. Ubx is known to control the McGinnis et al., 1984a,b; Sánchez-Herrero et al., 1985; Scott and expression of many genes that regulate tissue growth and patterning, Weiner, 1984; Struhl, 1982). but how it regulates tissue morphogenesis to reshape the wing into a In Drosophila, mutations in Ubx alter the identity of an entire haltere is still unclear. Here, we show that Ubx acts by repressing the segment of the body plan, namely transformation of the third expression of two genes in the haltere, Stubble and Notopleural, both thoracic segment into a duplicated second thoracic segment of which encode transmembrane proteases that remodel the apical (Bridges, 1944; Lewis, 1963, 1978, 1998). Ubx is strongly extracellular matrix to promote wing morphogenesis. -

The Overexpression of Homeotic Complex Gene Ultrabithorax in The
Bucknell University Bucknell Digital Commons Master’s Theses Student Theses 2010 The veo rexpression of homeotic complex gene Ultrabithorax in the post-embryonic neuronal lineages of the ventral nervous system in Drosophila melanogaster Katie Dry Bucknell University Follow this and additional works at: https://digitalcommons.bucknell.edu/masters_theses Part of the Medical Sciences Commons Recommended Citation Dry, Katie, "The vo erexpression of homeotic complex gene Ultrabithorax in the post-embryonic neuronal lineages of the ventral nervous system in Drosophila melanogaster" (2010). Master’s Theses. 14. https://digitalcommons.bucknell.edu/masters_theses/14 This Masters Thesis is brought to you for free and open access by the Student Theses at Bucknell Digital Commons. It has been accepted for inclusion in Master’s Theses by an authorized administrator of Bucknell Digital Commons. For more information, please contact [email protected]. ii ACKNOWLEDGMENTS I owe an incredible amount of gratitude to many people for this thesis coming to fruition. First, I would like to thank my graduate adviser, Dr. Elizabeth Marin, for welcoming me into her lab and bestowing upon me her vast knowledge of Drosophila neural development, genetics, and molecular techniques. Her patience and encouragement allowed me to proudly master a very complicated research project. Additionally, the results presented here would only be half complete without the determined and dependable work of my excellent labmate, Tony Cillo ’10. I have literally grown up at Bucknell University, arriving as a wide-eyed freshman and now leaving as a confident young adult. Certain I will find no other place like it, I will fondly carry Bucknell in my heart as I move forward. -

Ultrabithorax Is Essential for Bacteriocyte Development
Ultrabithorax is essential for bacteriocyte development Yu Matsuuraa,b,c, Yoshitomo Kikuchid, Toru Miurab, and Takema Fukatsua,c,1 aBioproduction Research Institute, National Institute of Advanced Industrial Science and Technology, Tsukuba 305-8566, Japan; bGraduate School of Environmental Science, Hokkaido University, Sapporo 060-0810, Japan; cGraduate School of Life and Environmental Sciences, University of Tsukuba, Tsukuba 305-8572, Japan; and dBioproduction Research Institute, National Institute of Advanced Industrial Science and Technology, Hokkaido Center, Sapporo 062-8517, Japan Edited by Nancy A. Moran, University of Texas at Austin, Austin, TX, and approved June 19, 2015 (received for review February 18, 2015) Symbiosis often entails the emergence of novel adaptive traits in transcription factors, in particular Ultrabithorax (Ubx), are involved organisms. Microbial symbionts are indispensable for diverse insects in the development of the host cells and organs specialized for via provisioning of essential nutrients, wherein novel host cells and harboring the symbiotic bacteria. organs for harboring the microbes, called bacteriocytes and bacter- iomes, have evolved repeatedly. Molecular and developmental mech- Results and Discussion anisms underpinning the emergence of novel symbiotic cells and Bacteriocyte Development During Embryogenesis of N. plebeius. We organs comprise an unsolved question in evolutionary developmental performed a detailed description of the embryogenesis of N. ple- biology. Here, we report that a conserved -

Wingless Signaling: a Genetic Journey from Morphogenesis to Metastasis
| FLYBOOK CELL SIGNALING Wingless Signaling: A Genetic Journey from Morphogenesis to Metastasis Amy Bejsovec1 Department of Biology, Duke University, Durham, North Carolina 27708 ORCID ID: 0000-0002-8019-5789 (A.B.) ABSTRACT This FlyBook chapter summarizes the history and the current state of our understanding of the Wingless signaling pathway. Wingless, the fly homolog of the mammalian Wnt oncoproteins, plays a central role in pattern generation during development. Much of what we know about the pathway was learned from genetic and molecular experiments in Drosophila melanogaster, and the core pathway works the same way in vertebrates. Like most growth factor pathways, extracellular Wingless/ Wnt binds to a cell surface complex to transduce signal across the plasma membrane, triggering a series of intracellular events that lead to transcriptional changes in the nucleus. Unlike most growth factor pathways, the intracellular events regulate the protein stability of a key effector molecule, in this case Armadillo/b-catenin. A number of mysteries remain about how the “destruction complex” desta- bilizes b-catenin and how this process is inactivated by the ligand-bound receptor complex, so this review of the field can only serve as a snapshot of the work in progress. KEYWORDS beta-catenin; FlyBook; signal transduction; Wingless; Wnt TABLE OF CONTENTS Abstract 1311 Introduction 1312 Introduction: Origin of the Wnt Name 1312 Discovery of the fly gene: the wingless mutant phenotype 1312 Discovery of wg homology to the mammalian oncogene int-1 1313 -

The Drosophila Microrna Iab-4 Causes a Dominant Homeotic Transformation of Halteres to Wings
Downloaded from genesdev.cshlp.org on October 6, 2021 - Published by Cold Spring Harbor Laboratory Press RESEARCH COMMUNICATION ample, miR-10 is located within the Drosophila Anten- The Drosophila microRNA napedia gene complex (ANT-C) between the Hox genes iab-4 causes a dominant Deformed and Sex combs reduced (Lagos-Quintana et al. 2001). The sequence and location of miR-10 are con- homeotic transformation of served in vertebrate Hox complexes (Tanzer et al. 2005). halteres to wings Sex combs reduced has been proposed as a direct miR-10 target in insects (Brennecke et al. 2005). A second group Matthew Ronshaugen,1 Frédéric Biemar,1 of Drosophila miRNAs map to a hairpin located at the 1 1,4 2,3 distal end of the iab-4 locus (Aravin et al. 2003), which Jessica Piel, Mike Levine, and Eric C. Lai resides between abd-A and Abd-B (Fig. 1A). miRNAs 1Department of Molecular and Cell Biology, Division of were cloned from both arms of this hairpin and are Genetics, Center for Integrative Genomics, University of termed iab-4–5p and iab-4–3p (Aravin et al. 2003). miR– California, Berkeley, California 94720, USA; 2Department of iab-4–5p was recently predicted to regulate Ubx activity Developmental Biology, Memorial Sloan-Kettering Cancer (Stark et al. 2003; Grun et al. 2005). Although vertebrates Center, New York, New York 10021, USA lack an iab-4 ortholog, as defined by sequence identity, a different miRNA, miR-196, resides at an analogous po- The Drosophila Bithorax Complex encodes three well- sition adjacent to the posterior-most HOX 9–13 paralogs. -

Wnt Signalling and Its Impact on Development and Cancer
PERS p ECTIVES Drosophila mutants was awarded the Nobel TIM e LIN e Prize in Physiology or Medicine in 1995 (TIMELINE). Today, the term Wnt is therefore Wnt signalling and its impact on an amalgam of Wg and Int10. There had been some earlier work on Wnt signalling, in ‘precloning’ times, when development and cancer the underlying pathways and mechanisms had not been identified. In the 1930s, viral Alexandra Klaus and Walter Birchmeier insertion was discovered to promote mam- mary tumours in laboratory mice (see Ref. 11 Abstract | The Wnt signalling pathway is an ancient system that has been highly for an example). Even earlier, in a famous conserved during evolution. It has a crucial role in the embryonic development experiment conducted in 1924, Mangold and of all animal species, in the regeneration of tissues in adult organisms and in Spemann grafted dorsal lips of the blastopore many other processes. Mutations or deregulated expression of components of from developing newt embryos onto the the Wnt pathway can induce disease, most importantly cancer. The first gene to opposing side of the embryo, inducing a 12 be identified that encodes a Wnt signalling component, Int1 (integration 1), was second body axis — a twin-headed embryo . The cause was the activity of the protein later molecularly characterized from mouse tumour cells 25 years ago. In parallel, the termed Wnt in the transplanted tissue frag- homologous gene Wingless in Drosophila melanogaster, which produces ment13. This work was awarded the Nobel developmental defects in embryos, was characterized. Since then, further Prize in 1935. Moreover, in an experiment components of the Wnt pathway have been identified and their epistatic performed in 1902 by Morgan, the simple salt relationships have been defined. -

The Fab-7 Element of the Bithorax Complex Attenuates Enhancer-Promoter Mteracnons in the Drosophila Embryo
Downloaded from genesdev.cshlp.org on October 4, 2021 - Published by Cold Spring Harbor Laboratory Press The Fab-7 element of the bithorax complex attenuates enhancer-promoter mteracnons in the Drosophila embryo Jumin Zhou, 1 Scott Barolo, 2 Paul Szymanski, 3 and Michael Levine 1'4 1Department of Molecular and Cellular Biology, Division of Genetics, University of California, Berkeley, California 94720-3201 USA; ~Departrnent of Biology, University of California at San Diego, La Jolla, California 92093 USA; 3Amplicon, Inc., East Setauket, New York 11733 USA Enhancers integrate positive and negative regulatory information to direct localized patterns of gene expression in the Drosophila embryo. Here we present evidence for the occurrence of cis regulatory elements that control the levels of gene expression by modulating enhancer-promoter interactions. For this purpose we have investigated the Drosophila bithorax complex (BX-C) because genetic studies suggest that the BX-C contains as much as 300 kb of cis regulatory information. A specialized DNA element, Fab-7, has been proposed to function as a boundary element that separates the iab-6 and iab-7 cis regulatory regions within the Abd-B domain of the BX-C. A 1.2-kb Fab-7 DNA fragment was placed between divergently transcribed white and lacZ test promoters and challenged with several defined enhancers expressed in the early embryo. These studies suggest that Fab-7 functions as an attenuator, which weakens gene expression by reducing enhancer-promoter interactions. Fab-7 selectively blocks distal enhancers in an orientation-independent fashion, and can function when located far from either the distal enhancer or target promoter.