An Energy Budget for the Olfactory Glomerulus
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Ultrastructural Study of the Granule Cell Domain of the Cochlear Nucleus in Rats: Mossy Fiber Endings and Their Targets
THE JOURNAL OF COMPARATIVE NEUROLOGY 369~345-360 ( 1996) Ultrastructural Study of the Granule Cell Domain of the Cochlear Nucleus in Rats: Mossy Fiber Endings and Their Targets DIANA L. WEEDMAN, TAN PONGSTAPORN, AND DAVID K. RYUGO Center for Hearing Sciences, Departments of Otolaryngoloby-Head and Neck Surgery and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, Maryland 2 1205 ABSTRACT The principal projection neurons of the cochlear nucleus receive the bulk of their input from the auditory nerve. These projection neurons reside in the core of the nucleus and are surrounded by an external shell, which is called the granule cell domain. Interneurons of the cochlear granule cell domain are the target for nonprimary auditory inputs, including projections from the superior olivary complex, inferior colliculus, and auditory cortex. The granule cell domain also receives projections from the cuneate and trigeminal nuclei, which are first-order nuclei of the somatosensory system. The cellular targets of the nonprimary projections are mostly unknown due to a lack of information regarding postsynaptic profiles in the granule cell areas. In the present paper, we examined the synaptic relationships between a heterogeneous class of large synaptic terminals called mossy fibers and their targets within subdivisions of the granule cell domain known as the lamina and superficial layer. By using light and electron microscopic methods in these subdivisions, we provide evidence for three different neuron classes that receive input from the mossy fibers: granule cells, unipolar brush cells, and a previously undescribed class called chestnut cells. The distinct synaptic relations between mossy fibers and members of each neuron class further imply fundamentally separate roles for processing acoustic signals. -

Distinct Representations of Olfactory Information in Different Cortical Centres
LETTER doi:10.1038/nature09868 Distinct representations of olfactory information in different cortical centres Dara L. Sosulski1, Maria Lissitsyna Bloom1{, Tyler Cutforth1{, Richard Axel1 & Sandeep Robert Datta1{ Sensory information is transmitted to the brain where it must be behaviours, but is unlikely to specify innate behaviours. Rather, innate processed to translate stimulus features into appropriate beha- olfactory behaviours are likely to result from the activation of genetically vioural output. In the olfactory system, distributed neural activity determined, stereotyped neural circuits. We have therefore developed a in the nose is converted into a segregated map in the olfactory strategy to trace the projections from identified glomeruli in the olfactory bulb1–3. Here we investigate how this ordered representation is bulb to higher olfactory cortical centres. transformed in higher olfactory centres in mice. We have Mitral and tufted cells that innervate a single glomerulus were developed a tracing strategy to define the neural circuits that labelled by electroporation of tetramethylrhodamine (TMR)-dextran convey information from individual glomeruli in the olfactory under the guidance of a two-photon microscope. This technique labels bulb to the piriform cortex and the cortical amygdala. The spatial mitral and tufted cells that innervate a single glomerulus and is suffi- order in the bulb is discarded in the piriform cortex; axons from ciently robust to allow the identification of axon termini within mul- individual glomeruli project diffusely to the piriform without tiple higher order olfactory centres (Figs 1a–c, 2 and Supplementary apparent spatial preference. In the cortical amygdala, we observe Figs 1–4). Labelling of glomeruli in the olfactory bulbs of mice that broad patches of projections that are spatially stereotyped for indi- express GFP under the control of specific odorant receptor promoters vidual glomeruli. -
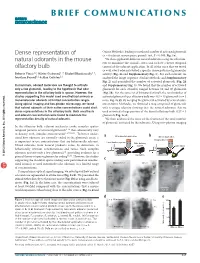
Dense Representation of Natural Odorants in the Mouse Olfactory Bulb
BRIEF COMMUNICATIONS Dense representation of Online Methods), leading to a reduced number of activated glomeruli (n = 6 odorant-mouse pairs, paired t test, P < 0.005; Fig. 1e). natural odorants in the mouse We then applied 40 different natural odorants using the olfactom- eter to minimize the animal’s stress and to have a better temporal olfactory bulb control of the odorant application. In all of the mice that we tested (n = 8), every odorant evoked a specific dense pattern of glomerular Roberto Vincis1,2, Olivier Gschwend1–3, Khaleel Bhaukaurally1–3, activity (Fig. 2a and Supplementary Fig. 1). For each odorant, we Jonathan Beroud1,2 & Alan Carleton1,2 analyzed the image sequence (Online Methods and Supplementary Fig. 2) and quantified the number of activated glomeruli (Fig. 2b In mammals, odorant molecules are thought to activate and Supplementary Fig. 3). We found that the number of activated only a few glomeruli, leading to the hypothesis that odor glomeruli for each stimulus ranged between 10 and 40 glomeruli representation in the olfactory bulb is sparse. However, the (Fig. 2b). For the same set of 30 natural stimuli, the total number of studies supporting this model used anesthetized animals or activated glomeruli per olfactory bulb was 443 ± 15 glomeruli (n = 5 monomolecular odorants at limited concentration ranges. mice; Fig. 2c,d). By merging the glomeruli activated by several odor- Using optical imaging and two-photon microscopy, we found ants (Online Methods), we obtained a map composed of glomeruli that natural odorants at their native concentrations could elicit with a unique identity showing that the natural odorants that we dense representations in the olfactory bulb. -

Functional Organization of Sensory Input to the Olfactory Bulb Glomerulus Analyzed by Two-Photon Calcium Imaging
Functional organization of sensory input to the olfactory bulb glomerulus analyzed by two-photon calcium imaging Matt Wachowiak*, Winfried Denk†, and Rainer W. Friedrich†‡ *Department of Biology, Boston University, 5 Cummington Street, Boston, MA 02215; and †Department of Biomedical Optics, Max Planck Institute for Medical Research, Jahnstrasse 29, D-69120 Heidelberg, Germany Edited by Charles F. Stevens, The Salk Institute for Biological Studies, La Jolla, CA, and approved May 10, 2004 (received for review January 20, 2004) Glomeruli in the olfactory bulb are anatomically discrete modules OSNs distinguished by histochemical markers terminate in dis- receiving input from idiotypic olfactory sensory neurons. To ex- crete subregions of the axonal compartment (36, 37). These data amine the functional organization of sensory inputs to individual raise the possibility that functionally distinct subdivisions exist .glomeruli, we loaded olfactory sensory neurons with a Ca2؉ within a glomerulus indicator and measured odorant-evoked presynaptic Ca2؉ signals Direct measurements of the activity of glomerular afferents within single glomeruli by using two-photon microscopy in anaes- have been difficult because most functional measures of intra- thetized mice. Odorants evoked patterns of discrete Ca2؉ signals glomerular activity lack sufficient spatial resolution or cellular throughout the neuropil of a glomerulus. Across glomeruli, Ca2؉ specificity. Here, we loaded OSNs with a fluorescent Ca2ϩ signals occurred with equal probability in all glomerular regions. indicator and measured patterns of afferent presynaptic activity ؉ Within single glomeruli, the pattern of intraglomerular Ca2 sig- within glomeruli by two-photon Ca2ϩ imaging in anaesthetized nals was indistinguishable for stimuli of different duration, iden- mice. Our data indicate that OSN input to individual glomeruli tity, and concentration. -

Medial Temporal Lobe (The Limbic System)
MEDIAL TEMPORAL LOBE (THE LIMBIC SYSTEM) On the medial surface of the temporal lobe are three structures critical for normal human functioning. From rostral to caudal, they are the olfactory cortex, the amygdala, and the hippocampus. We will look at the anatomy and function of each separately, although they are often grouped together as "the limbic system". A. The olfactory system: The olfactory system actually begins in the roof of the nasal cavity. The olfactory receptors are ciliated epithelial cells with an array of receptors capable of detecting thousands of different odors. However, just as with any sensory system, the receptor neurons themselves do not project to the cerebral hemispheres. Their axons project up through the cribiform plate of the skull to synapse on the dendrites of the mitral cells of the olfactory bulb. The axons of the olfactory receptors make up the elusive cranial nerve I. This fragile tract is susceptible to shearing forces in head trauma, and loss of smell is a surprisingly debilitating injury. Here is an example of a section through olfactory bulb. The olfactory bulb is not a simple relay (something which passively transmits the signal), but is a sophisticated structure in itself. The mitral cell- olfactory neuron synapse is actually within a tangle of axons and dendrites that is called a glomerulus. There is a second cell type tucked around these glomeruli which probably affects how the signal is transmitted. These cells are small and densely packed, which gives them the name "granule cells". However, they bear no relation to the granule cells of the cerebellum or cerebral cortex. -

Granule Cell Synapses of Rat Cerebellum: Experimental Observations and Theoretical Predictions
Articles in PresS. J Neurophysiol (October 5, 2005). doi:10.1152/jn.00696.2005 1 LTP regulates burst initiation and frequency at mossy fiber – granule cell synapses of rat cerebellum: experimental observations and theoretical predictions Thierry Nieus1,2, Elisabetta Sola1,4, Jonathan Mapelli1, Elena Saftenku3, Paola Rossi1, Egidio D’Angelo1,2,* 1 Dept. of Cellular-Molecular Physiological and Pharmacological Sciences (University of Pavia) and INFM, Via Forlanini 6, I-27100, Pavia, Italy. 2 Dept. of Functional and Evolutionary Biology (University of Parma), Parco Area delle Scienze 11a, I-34100, Parma, Italy. 3 Dept. of General Physiology of Nervous System, A.A. Bogomoletz Institute of Physiology Bogomoletz St., 4, 01024, Kiev-24, Ukraine 4 present address: Dept. of Biophysics, SISSA, via Beirut 4, I-34014, Trieste, Italy * To whom correspondence should be addressed at [email protected] Key-words. LTP, Synaptic plasticity, Presynaptic mechanisms, Cerebellum, Granule cells Running title. LTP and bursting in the cerebellum Acknowledgments. This work was supported by projects of the European Community (CEREBELLUM QLG3-CT-2001-02256 and SPIKEFORCE IST-2001-35271), of MIUR and INFM of Italy to ED. ABSTRACT Long-term potentiation (LTP) is a synaptic change supposed to provide the cellular basis for learning and memory in brain neuronal circuits. Although specific LTP expression mechanisms could be critical to determine the dynamics of repetitive neurotransmission, this important issue remained largely unexplored. In this paper we have performed whole-cell patch-clamp recordings of mossy fiber – granule cell LTP in acute rat cerebellar slices and investigated its computational implications with a mathematical model. During LTP, stimulation with short impulse trains at 100 Hz revealed earlier initiation of granule cell spike bursts and a smaller non-significant spike frequency increase. -

Taste and Smell Disorders in Clinical Neurology
TASTE AND SMELL DISORDERS IN CLINICAL NEUROLOGY OUTLINE A. Anatomy and Physiology of the Taste and Smell System B. Quantifying Chemosensory Disturbances C. Common Neurological and Medical Disorders causing Primary Smell Impairment with Secondary Loss of Food Flavors a. Post Traumatic Anosmia b. Medications (prescribed & over the counter) c. Alcohol Abuse d. Neurodegenerative Disorders e. Multiple Sclerosis f. Migraine g. Chronic Medical Disorders (liver and kidney disease, thyroid deficiency, Diabetes). D. Common Neurological and Medical Disorders Causing a Primary Taste disorder with usually Normal Olfactory Function. a. Medications (prescribed and over the counter), b. Toxins (smoking and Radiation Treatments) c. Chronic medical Disorders ( Liver and Kidney Disease, Hypothyroidism, GERD, Diabetes,) d. Neurological Disorders( Bell’s Palsy, Stroke, MS,) e. Intubation during an emergency or for general anesthesia. E. Abnormal Smells and Tastes (Dysosmia and Dysgeusia): Diagnosis and Treatment F. Morbidity of Smell and Taste Impairment. G. Treatment of Smell and Taste Impairment (Education, Counseling ,Changes in Food Preparation) H. Role of Smell Testing in the Diagnosis of Neurodegenerative Disorders 1 BACKGROUND Disorders of taste and smell play a very important role in many neurological conditions such as; head trauma, facial and trigeminal nerve impairment, and many neurodegenerative disorders such as Alzheimer’s, Parkinson Disorders, Lewy Body Disease and Frontal Temporal Dementia. Impaired smell and taste impairs quality of life such as loss of food enjoyment, weight loss or weight gain, decreased appetite and safety concerns such as inability to smell smoke, gas, spoiled food and one’s body odor. Dysosmia and Dysgeusia are very unpleasant disorders that often accompany smell and taste impairments. -

Three-Dimensional Morphology of Cerebellar Protoplasmic Islands
Scanning Microscopy Volume 5 Number 2 Article 16 2-16-1991 Three-Dimensional Morphology of Cerebellar Protoplasmic Islands and Proteoglycan Content of Mossy Fiber Glomerulus: A Scanning and Transmission Electron Microscope Study O. J. Castejón Universidad del Zulia, Venezuela H. V. Castejón Universidad del Zulia, Venezuela Follow this and additional works at: https://digitalcommons.usu.edu/microscopy Part of the Biology Commons Recommended Citation Castejón, O. J. and Castejón, H. V. (1991) "Three-Dimensional Morphology of Cerebellar Protoplasmic Islands and Proteoglycan Content of Mossy Fiber Glomerulus: A Scanning and Transmission Electron Microscope Study," Scanning Microscopy: Vol. 5 : No. 2 , Article 16. Available at: https://digitalcommons.usu.edu/microscopy/vol5/iss2/16 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Scanning Microscopy by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Scanning Microscopy , Vol. 5, No . 2, 1991 (Pages 477-494) 0891 -7035/91$3.00+ .00 Scanning Microscopy International , Chicago (AMF O'Hare) , IL 60666 USA THREE-DI MENSIONAL MORPHOLOGYOF CEREBELLAR PROTOPLASMIC ISLANDS AND PROTEOGLYCANCO NTENT OF MOSSY FIBER GLOMERULUS: A SCANNING AND TRANSMISSION ELECTRON MICROSCOPE STUDY 0. J . Cas tejon and H. V. Castejon Institute de Investigac1ones Biologicas. Facultad de Medicina . Universidad de l Zulia. Apart ado 526, Mara ca ibo, Venezu ela (Received for publication May 2, 1990 , and in revised form February 16, 1991) Abstract Introduction The present rev , ew summarizes the outer and The cerebellar protoplasmatic islands and inner surface features of mossy f ib er glomeru li the cerebellar glomeruli were studied at light 1n vertebra te cerebellar granular la yer as seen microscope level by Denissenko ( 18771, Ramon y by conv en t i ona l scanning electron microscopy Cajal ( 1889, 19551, Retzius ( 1892a,b), Lugaro <SEMI and SEM fre eze -f ra cture method . -

Astroglial Glutamate Transporters in the Brain: Regulating Neurotransmitter Homeostasis and Synaptic Transmission
Journal of Neuroscience Research 95:2140–2151 (2017) Review Astroglial Glutamate Transporters in the Brain: Regulating Neurotransmitter Homeostasis and Synaptic Transmission Ciaran Murphy-Royal,1,2 Julien Dupuis,2,3 Laurent Groc,2,3 and Stephane H. R. Oliet 1,2 1Neurocentre Magendie, Inserm U1215, Bordeaux, France 2Universite de Bordeaux, Bordeaux, France 3Interdisciplinary Institute for Neuroscience, CNRS UMR 5297, Bordeaux, France Astrocytes, the major glial cell type in the central nervous compartments. As an example, the extracellular glutamate system (CNS), are critical for brain function and have concentration is maintained at low values lying most like- been implicated in various disorders of the central ner- ly below 100 nM (Hamberger and Nystrom,€ 1984; vous system. These cells are involved in a wide range of Cavelier and Attwell, 2005; Herman and Jahr, 2007), cerebral processes including brain metabolism, control of while it is much more concentrated inside cells. As there central blood flow, ionic homeostasis, fine-tuning synap- are no extracellular enzymes degrading glutamate, this tic transmission, and neurotransmitter clearance. Such low extracellular concentration can be fully accounted for varied roles can be efficiently carried out due to the inti- by specific transporters, which are widely expressed mate interactions astrocytes maintain with neurons, the throughout the brain (Furuta et al., 1997). Glutamate vasculature, as well as with other glial cells. Arguably, one transporters working under physiological conditions have of the most important functions of astrocytes in the brain the capacity to establish concentration gradients of 106 is their control of neurotransmitter clearance. This is across the plasma membrane, theoretically resulting in a particularly true for glutamate whose timecourse in the glutamate concentration of 10 nM extracellularly and 10 synaptic cleft needs to be controlled tightly under physio- mM intracellularly (Zerangue and Kavanaugh, 1996a). -

Svetla P. Pencheva, Emilyan A. Ivanov Summary ULTRASTRUCTURAL
J Biomed Clin Res Volume 6 Number 1, 2013 DOI: 10.1515/jbcr-2015-0095 Original Article ULTRASTRUCTURAL STUDY OF THE SYNAPTIC GLOMERULI IN THE RAT'S COCHLEAR NUCLEUS Svetla P. Pencheva, Summary Emilyan A. Ivanov The granule cell domain of the cochlear nuclear complex Department of Anatomy, Histology, contains interneurons, which are the targets for Citology and Biology nonprimary auditory inputs from the superior olivary complex, inferior colliculus, auditory cortex, cuneate and Medical University – Pleven trigeminal nuclei of the somatosensory system. The Bulgaria cellular targets of the non-primary projections are unknown due to a lack of information regarding postsynaptic profiles in the granule cell areas. In the present paper, we examined the synaptic relationships between a heterogeneous class of large synaptic terminals, called mossy fibers and their targets within subdivisions of the granule cell domain. During the late stage of postnatal development, we observed heterogenous groups of complex synaptic glomeruli. Using electron microscopy, we provide evidence for ultrastructural features of dendrites that receive input from the mossy fibers. The distinct synaptic relations between mossy fibers and dendrites of microneurons further imply fundamentally separate roles in processing of acoustic signals. Key wards: cochlear nucleus, granule cell domain, synaptic glomerulus Introduction The granule cell domain of the cochlear nucleus Corresponding Autor: comprises up to seven different subdivisions [1]. Svetla P. Pencheva These subdivisions are found around the core of the Sector of Anatomy, Histology and ventral cochlear nucleus (VCN) and the dorsal Cytology cochlear nucleus ( DCN), including the superficial Medical University-Pleven layer of the VCN, the lamina dividing DCN from Pleven, 5800 Bulgaria VCN, the subpeduncular dorsal corner of the VCN, е-mail: [email protected] the dorsal strial corner of the DCN, and layer II of the DCN [2, 3]. -

Asymmetric Neurotransmitter Release Enables Rapid Odour Lateralization in Drosophila
SUPPLEMENTARY INFORMATION doi:10.1038/nature11747 SUPPLEMENTARY NOTES Note S1: Light offset produced a precise compensatory turn in the direction opposite to the light-evoked turn (Fig. 1g). As a result, the fly’s net lateral displacement after this compensatory turn was close to zero (Fig. 1i). A train of light pulses produced a larger turn, and the fly’s compensatory turn after light offset was commensurately larger, although less precise (Fig. 1i). This suggests that the fly keeps track of how far it has turned away from its original path, and the accuracy of its estimate depends on how far it has strayed. Note S2: Resting GCaMP fluorescence (F) was not significantly different in the right and left glomeruli (p = 0.67, n = 6, paired t-test). This is expected because each glomerulus contains the axons arising from both the right and left antennae. Although one antenna is removed, the cut axons arising from that antenna are still present in the neuropil. Thus, both right and left glomeruli must contain the same number of GCaMP molecules, and so resting fluorescence is the same. Spontaneous ORN activity evidently makes little contribution to F. What is the mechanistic basis for the significant ipsi/contra difference in F/F? A previous study reported that an ORN plasma membrane marker is two-fold more abundant in the ipsilateral glomerulus as compared to the contralateral glomerulus1. This suggests that the ipsilateral arbor is simply larger than the contralateral arbor of the same ORN. If the properties of ipsi- and contralateral branches are the same, then the ipsi/contra asymmetry in F/F should be roughly proportional to the ipsi/contra asymmetry in arbor size. -

Morphogenetic Plasticity of Neuronal Elements in Cerebellar Glomeruli During Deafferentation-Induced Synaptic Reorganization J6zsefhhmori, Robert L
(C)Freund Publishing House Ltd., 1997 Morphogenetic Plasticity of Neuronal Elements in Cerebellar Glomeruli during Deafferentation-Induced Synaptic Reorganization J6zsefHhmori, Robert L. Jakab and J6zsefTakb.cs Department ofAnatomy, Laboratory ofNeurobiology, Semmelweis University, Medical School, Budapest, Hungary, H-1094; Section ofNeurobiology, Yale University School ofMedicine, New Haven, CT, USA SUMMARY represented only one-fifth of all synaptic junctions. The quantitative data of the Reorganization of the cerebellar glomerulus, reorganized cerebellar glomerulus demonstrate the main synaptic complex within the granule both a remarkable constancy and a plasticity of cell layer, was investigated using quantitative the excitatory granule cells and inhibitory Golgi morphological techniques. All afferents to the neurons building up this synaptic complex. cerebellar cortex, including mossy-fibers, were Constancy (the preservation of certain specific surgically destroyed by undercutting the structural features) is represented by an cerebellar vermis. Fifteen days after the eventually unchanged number of dendrites and operation, which resulted in the removal of the synaptic junctions within the deafferented main excitatory afferent to the glomerulus, a glomerulus. Such constancy was made possible, significant reorganization of the whole synaptic however, by the morphogenetic plasticity of complex was observed, whereas the structural both nerve-cell types to produce new, dendro- integrity of the glomerulus was remarkably well dendritic and axo-dendritic synapses to preserved. This was indicated by the compensate for the loss of mossy-fiber synapses. observation that the number of granule cell dendrites per glomerulus), as well as the (50 KEY number of dendritic digits (210 per WORDS glomerulus) bearing most of the 230 synaptic eerebellar cortex, deafferentation, synaptic junctions per glomerulus, did not change EM significantly after mossy-fiber degeneration.