Amygdala Corticofugal Input Shapes Mitral Cell Responses in the Accessory Olfactory Bulb
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Born Granule Cells During Implicit Versus Explicit Olfactory Learning
RESEARCH ARTICLE Opposite regulation of inhibition by adult- born granule cells during implicit versus explicit olfactory learning Nathalie Mandairon1*, Nicola Kuczewski1, Florence Kermen1, Je´ re´ my Forest1, Maellie Midroit1, Marion Richard1, Marc Thevenet1, Joelle Sacquet1, Christiane Linster2,3, Anne Didier1 1Lyon Neuroscience Research Center, Neuroplasticity and Neuropathology of Olfactory Perception Team, CNRS UMR 5292, INSERM U1028, Universite´ de Lyon, Lyon, France; 2Computational Physiology Lab, Cornell University, Ithaca, United States; 3Department of Neurobiology and Behavior, Cornell University, Ithaca, United States Abstract Both passive exposure and active learning through reinforcement enhance fine sensory discrimination abilities. In the olfactory system, this enhancement is thought to occur partially through the integration of adult-born inhibitory interneurons resulting in a refinement of the representation of overlapping odorants. Here, we identify in mice a novel and unexpected dissociation between passive and active learning at the level of adult-born granule cells. Specifically, while both passive and active learning processes augment neurogenesis, adult-born cells differ in their morphology, functional coupling and thus their impact on olfactory bulb output. Morphological analysis, optogenetic stimulation of adult-born neurons and mitral cell recordings revealed that passive learning induces increased inhibitory action by adult-born neurons, probably resulting in more sparse and thus less overlapping odor representations. Conversely, after active learning inhibitory action is found to be diminished due to reduced connectivity. In this case, strengthened odor response might underlie enhanced discriminability. *For correspondence: DOI: https://doi.org/10.7554/eLife.34976.001 [email protected] Competing interests: The authors declare that no Introduction competing interests exist. Brain representations of the environment constantly evolve through learning mediated by different Funding: See page 13 plasticity mechanisms. -

Cranial Nerves
Cranial Nerves (1,5,7,8,9,10,11 and 12) Slides not included 9th and 10th Cranial 11th and 12th Cranial 8th Cranial Nerve 5th and 7th Cranial 1st Cranial Nerve Nerves Nerves Nerves (3,7,11,12,13,21,23,24) - (10,16) (12,23) Slides included: (14 to 17) *Slides that are not included mostly are slides of summaries or pictures. Nouf Alabdulkarim. Med 435 Olfactory Nerve [The 1st Cranial Nerve] Special Sensory Olfactory pathway 1st order neuron Receptors Axons of 1st order Neurons Olfactory receptors are specialized, ciliated nerve cells The axons of these bipolar cells 12 -20 fibers form the that lie in the olfactory epithelium. true olfactory nerve fibers. Which passes through the cribriform plate of ethmoid → They join the olfactory bulb Preliminary processing of olfactory information It is within the olfactory bulb, which contains interneurones and large Mitral cells; axons from the latter leave the bulb to form the olfactory tract. nd 2 order neuron • It is formed by the Mitral cells of olfactory bulb. • The axons of these cells form the olfactory tract. • Each tract divides into 2 roots at the anterior perforated substance: Lateral root Medial root Carries olfactory fibers to end in cortex of the Uncus & • crosses midline through anterior commissure adjacent part of Hippocampal gyrus (center of smell). and joins the uncrossed lateral root of opposite side. • It connects olfactory centers of 2 cerebral hemispheres. • So each olfactory center receives smell sensation from both halves of nasal cavity. NB. Olfactory pathway is the only sensory pathway which reaches the cerebral cortex without passing through the Thalamus . -

Distinct Representations of Olfactory Information in Different Cortical Centres
LETTER doi:10.1038/nature09868 Distinct representations of olfactory information in different cortical centres Dara L. Sosulski1, Maria Lissitsyna Bloom1{, Tyler Cutforth1{, Richard Axel1 & Sandeep Robert Datta1{ Sensory information is transmitted to the brain where it must be behaviours, but is unlikely to specify innate behaviours. Rather, innate processed to translate stimulus features into appropriate beha- olfactory behaviours are likely to result from the activation of genetically vioural output. In the olfactory system, distributed neural activity determined, stereotyped neural circuits. We have therefore developed a in the nose is converted into a segregated map in the olfactory strategy to trace the projections from identified glomeruli in the olfactory bulb1–3. Here we investigate how this ordered representation is bulb to higher olfactory cortical centres. transformed in higher olfactory centres in mice. We have Mitral and tufted cells that innervate a single glomerulus were developed a tracing strategy to define the neural circuits that labelled by electroporation of tetramethylrhodamine (TMR)-dextran convey information from individual glomeruli in the olfactory under the guidance of a two-photon microscope. This technique labels bulb to the piriform cortex and the cortical amygdala. The spatial mitral and tufted cells that innervate a single glomerulus and is suffi- order in the bulb is discarded in the piriform cortex; axons from ciently robust to allow the identification of axon termini within mul- individual glomeruli project diffusely to the piriform without tiple higher order olfactory centres (Figs 1a–c, 2 and Supplementary apparent spatial preference. In the cortical amygdala, we observe Figs 1–4). Labelling of glomeruli in the olfactory bulbs of mice that broad patches of projections that are spatially stereotyped for indi- express GFP under the control of specific odorant receptor promoters vidual glomeruli. -

The Connexions of the Amygdala
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.28.2.137 on 1 April 1965. Downloaded from J. Neurol. Neurosurg. Psychiat., 1965, 28, 137 The connexions of the amygdala W. M. COWAN, G. RAISMAN, AND T. P. S. POWELL From the Department of Human Anatomy, University of Oxford The amygdaloid nuclei have been the subject of con- to what is known of the efferent connexions of the siderable interest in recent years and have been amygdala. studied with a variety of experimental techniques (cf. Gloor, 1960). From the anatomical point of view MATERIAL AND METHODS attention has been paid mainly to the efferent connexions of these nuclei (Adey and Meyer, 1952; The brains of 26 rats in which a variety of stereotactic or Lammers and Lohman, 1957; Hall, 1960; Nauta, surgical lesions had been placed in the diencephalon and and it is now that there basal forebrain areas were used in this study. Following 1961), generally accepted survival periods of five to seven days the animals were are two main efferent pathways from the amygdala, perfused with 10 % formol-saline and after further the well-known stria terminalis and a more diffuse fixation the brains were either embedded in paraffin wax ventral pathway, a component of the longitudinal or sectioned on a freezing microtome. All the brains were association bundle of the amygdala. It has not cut in the coronal plane, and from each a regularly spaced generally been recognized, however, that in studying series was stained, the paraffin sections according to the Protected by copyright. the efferent connexions of the amygdala it is essential original Nauta and Gygax (1951) technique and the frozen first to exclude a contribution to these pathways sections with the conventional Nauta (1957) method. -
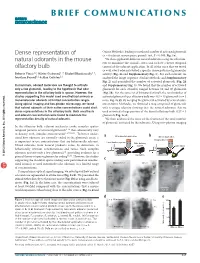
Dense Representation of Natural Odorants in the Mouse Olfactory Bulb
BRIEF COMMUNICATIONS Dense representation of Online Methods), leading to a reduced number of activated glomeruli (n = 6 odorant-mouse pairs, paired t test, P < 0.005; Fig. 1e). natural odorants in the mouse We then applied 40 different natural odorants using the olfactom- eter to minimize the animal’s stress and to have a better temporal olfactory bulb control of the odorant application. In all of the mice that we tested (n = 8), every odorant evoked a specific dense pattern of glomerular Roberto Vincis1,2, Olivier Gschwend1–3, Khaleel Bhaukaurally1–3, activity (Fig. 2a and Supplementary Fig. 1). For each odorant, we Jonathan Beroud1,2 & Alan Carleton1,2 analyzed the image sequence (Online Methods and Supplementary Fig. 2) and quantified the number of activated glomeruli (Fig. 2b In mammals, odorant molecules are thought to activate and Supplementary Fig. 3). We found that the number of activated only a few glomeruli, leading to the hypothesis that odor glomeruli for each stimulus ranged between 10 and 40 glomeruli representation in the olfactory bulb is sparse. However, the (Fig. 2b). For the same set of 30 natural stimuli, the total number of studies supporting this model used anesthetized animals or activated glomeruli per olfactory bulb was 443 ± 15 glomeruli (n = 5 monomolecular odorants at limited concentration ranges. mice; Fig. 2c,d). By merging the glomeruli activated by several odor- Using optical imaging and two-photon microscopy, we found ants (Online Methods), we obtained a map composed of glomeruli that natural odorants at their native concentrations could elicit with a unique identity showing that the natural odorants that we dense representations in the olfactory bulb. -

Functional Organization of Sensory Input to the Olfactory Bulb Glomerulus Analyzed by Two-Photon Calcium Imaging
Functional organization of sensory input to the olfactory bulb glomerulus analyzed by two-photon calcium imaging Matt Wachowiak*, Winfried Denk†, and Rainer W. Friedrich†‡ *Department of Biology, Boston University, 5 Cummington Street, Boston, MA 02215; and †Department of Biomedical Optics, Max Planck Institute for Medical Research, Jahnstrasse 29, D-69120 Heidelberg, Germany Edited by Charles F. Stevens, The Salk Institute for Biological Studies, La Jolla, CA, and approved May 10, 2004 (received for review January 20, 2004) Glomeruli in the olfactory bulb are anatomically discrete modules OSNs distinguished by histochemical markers terminate in dis- receiving input from idiotypic olfactory sensory neurons. To ex- crete subregions of the axonal compartment (36, 37). These data amine the functional organization of sensory inputs to individual raise the possibility that functionally distinct subdivisions exist .glomeruli, we loaded olfactory sensory neurons with a Ca2؉ within a glomerulus indicator and measured odorant-evoked presynaptic Ca2؉ signals Direct measurements of the activity of glomerular afferents within single glomeruli by using two-photon microscopy in anaes- have been difficult because most functional measures of intra- thetized mice. Odorants evoked patterns of discrete Ca2؉ signals glomerular activity lack sufficient spatial resolution or cellular throughout the neuropil of a glomerulus. Across glomeruli, Ca2؉ specificity. Here, we loaded OSNs with a fluorescent Ca2ϩ signals occurred with equal probability in all glomerular regions. indicator and measured patterns of afferent presynaptic activity ؉ Within single glomeruli, the pattern of intraglomerular Ca2 sig- within glomeruli by two-photon Ca2ϩ imaging in anaesthetized nals was indistinguishable for stimuli of different duration, iden- mice. Our data indicate that OSN input to individual glomeruli tity, and concentration. -

Medial Temporal Lobe (The Limbic System)
MEDIAL TEMPORAL LOBE (THE LIMBIC SYSTEM) On the medial surface of the temporal lobe are three structures critical for normal human functioning. From rostral to caudal, they are the olfactory cortex, the amygdala, and the hippocampus. We will look at the anatomy and function of each separately, although they are often grouped together as "the limbic system". A. The olfactory system: The olfactory system actually begins in the roof of the nasal cavity. The olfactory receptors are ciliated epithelial cells with an array of receptors capable of detecting thousands of different odors. However, just as with any sensory system, the receptor neurons themselves do not project to the cerebral hemispheres. Their axons project up through the cribiform plate of the skull to synapse on the dendrites of the mitral cells of the olfactory bulb. The axons of the olfactory receptors make up the elusive cranial nerve I. This fragile tract is susceptible to shearing forces in head trauma, and loss of smell is a surprisingly debilitating injury. Here is an example of a section through olfactory bulb. The olfactory bulb is not a simple relay (something which passively transmits the signal), but is a sophisticated structure in itself. The mitral cell- olfactory neuron synapse is actually within a tangle of axons and dendrites that is called a glomerulus. There is a second cell type tucked around these glomeruli which probably affects how the signal is transmitted. These cells are small and densely packed, which gives them the name "granule cells". However, they bear no relation to the granule cells of the cerebellum or cerebral cortex. -

Taste and Smell Disorders in Clinical Neurology
TASTE AND SMELL DISORDERS IN CLINICAL NEUROLOGY OUTLINE A. Anatomy and Physiology of the Taste and Smell System B. Quantifying Chemosensory Disturbances C. Common Neurological and Medical Disorders causing Primary Smell Impairment with Secondary Loss of Food Flavors a. Post Traumatic Anosmia b. Medications (prescribed & over the counter) c. Alcohol Abuse d. Neurodegenerative Disorders e. Multiple Sclerosis f. Migraine g. Chronic Medical Disorders (liver and kidney disease, thyroid deficiency, Diabetes). D. Common Neurological and Medical Disorders Causing a Primary Taste disorder with usually Normal Olfactory Function. a. Medications (prescribed and over the counter), b. Toxins (smoking and Radiation Treatments) c. Chronic medical Disorders ( Liver and Kidney Disease, Hypothyroidism, GERD, Diabetes,) d. Neurological Disorders( Bell’s Palsy, Stroke, MS,) e. Intubation during an emergency or for general anesthesia. E. Abnormal Smells and Tastes (Dysosmia and Dysgeusia): Diagnosis and Treatment F. Morbidity of Smell and Taste Impairment. G. Treatment of Smell and Taste Impairment (Education, Counseling ,Changes in Food Preparation) H. Role of Smell Testing in the Diagnosis of Neurodegenerative Disorders 1 BACKGROUND Disorders of taste and smell play a very important role in many neurological conditions such as; head trauma, facial and trigeminal nerve impairment, and many neurodegenerative disorders such as Alzheimer’s, Parkinson Disorders, Lewy Body Disease and Frontal Temporal Dementia. Impaired smell and taste impairs quality of life such as loss of food enjoyment, weight loss or weight gain, decreased appetite and safety concerns such as inability to smell smoke, gas, spoiled food and one’s body odor. Dysosmia and Dysgeusia are very unpleasant disorders that often accompany smell and taste impairments. -

Smell & Taste.Pdf
Smell and Taste 428 Special senses 1. SMELL (OLFACTION) 1.1 Overview Smell is the least Understood sense. It is mainly subjective. In dogs and other animals, it is more developed than humans. - There are dfferent stimuli that can be smelled such as: camphoraceous, musky, flora (flower), pepperminty, ethereal, pungent, putrid 1.2 Structure of Olfactory epithelium and bulb See the figure on the next page! 1.2.1 Olfactory mucous membrane It is the upper lining of the nasal cavity (near the septum), containing olfactory (odorant) receptors that are responsible for smelling. o Olfactory receptors are bipolar neurons which receive stimuli in the nasal cavity (through cilia) and transmits them through axons, leave the olfactory epithelium and travel into CNS (olfactory bulb). o Although they are nerve cells, olfactory receptor cells are replaced every 60 days or so, and they grow their axon into the correct place in CNS. Olfactory epithelium contains three types of cells (the olfactory receptors cells discussed) as well as two other types of cells: o Olfactory (Bowman’s) glands: produce mucus that dissolves odorants o Supporting cell o Basal cells: regenerate olfactory receptor cells. 1 Smell and Taste 428 1.2.2 Olfactory bulb The olfactory bulb is made up of nerves that receive olfactory signals from axons of olfactory receptor cells. These nerves are of two cell types: o Mitral cells (most important) (M) o Tufted cells (smaller than mitral cells) (T) Mitral and tufted cells release glutamate The synapse between the axons of olfactory receptor cells and dendrites of mitral cells occur in clusters called olphactory glomeruli (OG) In a glomerulus, about 1000 olfactory receptor axons converge onto 1 mitral cell. -

Rhesus Monkey Brain Atlas Subcortical Gray Structures
Rhesus Monkey Brain Atlas: Subcortical Gray Structures Manual Tracing for Hippocampus, Amygdala, Caudate, and Putamen Overview of Tracing Guidelines A) Tracing is done in a combination of the three orthogonal planes, as specified in the detailed methods that follow. B) Each region of interest was originally defined in the right hemisphere. The labels were then reflected onto the left hemisphere and all borders checked and adjusted manually when necessary. C) For the initial parcellation, the user used the “paint over function” of IRIS/SNAP on the T1 template of the atlas. I. Hippocampus Major Boundaries Superior boundary is the lateral ventricle/temporal horn in the majority of slices. At its most lateral extent (subiculum) the superior boundary is white matter. The inferior boundary is white matter. The anterior boundary is the lateral ventricle/temporal horn and the amygdala; the posterior boundary is lateral ventricle or white matter. The medial boundary is CSF at the center of the brain in all but the most posterior slices (where the medial boundary is white matter). The lateral boundary is white matter. The hippocampal trace includes dentate gyrus, the CA3 through CA1 regions of the hippocamopus, subiculum, parasubiculum, and presubiculum. Tracing A) Tracing is done primarily in the sagittal plane, working lateral to medial a. Locate the most lateral extent of the subiculum, which is bounded on all sides by white matter, and trace. b. As you page medially, tracing the hippocampus in each slice, the superior, anterior, and posterior boundaries of the hippocampus become the lateral ventricle/temporal horn. c. Even further medially, the anterior boundary becomes amygdala and the posterior boundary white matter. -

Odour Discrimination Learning in the Indian Greater Short-Nosed Fruit Bat
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb175364. doi:10.1242/jeb.175364 RESEARCH ARTICLE Odour discrimination learning in the Indian greater short-nosed fruit bat (Cynopterus sphinx): differential expression of Egr-1, C-fos and PP-1 in the olfactory bulb, amygdala and hippocampus Murugan Mukilan1, Wieslaw Bogdanowicz2, Ganapathy Marimuthu3 and Koilmani Emmanuvel Rajan1,* ABSTRACT transferred directly from the olfactory bulb to the amygdala and Activity-dependent expression of immediate-early genes (IEGs) is then to the hippocampus (Wilson et al., 2004; Mouly and induced by exposure to odour. The present study was designed to Sullivan, 2010). Depending on the context, the learning investigate whether there is differential expression of IEGs (Egr-1, experience triggers neurotransmitter release (Lovinger, 2010) and C-fos) in the brain region mediating olfactory memory in the Indian activates a signalling cascade through protein kinase A (PKA), greater short-nosed fruit bat, Cynopterus sphinx. We assumed extracellular signal-regulated kinase-1/2 (ERK-1/2) (English and that differential expression of IEGs in different brain regions may Sweatt, 1997; Yoon and Seger, 2006; García-Pardo et al., 2016) and orchestrate a preference odour (PO) and aversive odour (AO) cyclic AMP-responsive element binding protein-1 (CREB-1), memory in C. sphinx. We used preferred (0.8% w/w cinnamon which is phosphorylated by ERK-1/2 (Peng et al., 2010). powder) and aversive (0.4% w/v citral) odour substances, with freshly Activated CREB-1 induces expression of immediate-early genes prepared chopped apple, to assess the behavioural response and (IEGs), such as early growth response gene-1 (Egr-1) (Cheval et al., induction of IEGs in the olfactory bulb, hippocampus and amygdala. -

Arc-Expressing Neuronal Ensembles
14070 • The Journal of Neuroscience, October 14, 2015 • 35(41):14070–14075 Brief Communications Arc-Expressing Neuronal Ensembles Supporting Pattern Separation Require Adrenergic Activity in Anterior Piriform Cortex: An Exploration of Neural Constraints on Learning X Amin MD. Shakhawat,1 XAli Gheidi,1 X Iain T. MacIntyre,1 Melissa L. Walsh,1 XCarolyn W. Harley,2 and XQi Yuan1 1Division of Biomedical Sciences, Faculty of Medicine, and 2Department of Psychology, Faculty of Science, Memorial University of Newfoundland, St. John’s, Newfoundland A1B 3V6, Canada Arc ensembles in adult rat olfactory bulb (OB) and anterior piriform cortex (PC) were assessed after discrimination training on highly similar odor pairs. Nonselective ␣- and -adrenergic antagonists or saline were infused in the OB or anterior PC during training. OB adrenergic blockade slowed, but did not prevent, odor discrimination learning. After criterion performance, Arc ensembles in anterior piriform showed enhanced stability for the rewarded odor and pattern separation for the discriminated odors as described previously. Anterior piriform adrenergic blockade prevented acquisition of similar odor discrimination and of OB ensemble changes, even with extended overtraining. Mitral and granule cell Arc ensembles in OB showed enhanced stability for rewarded odor only in the saline group. Pattern separation was not seen in the OB. Similar odor discrimination co-occurs with increased stability in rewarded odor representa- tions and pattern separation to reduce encoding overlap. The difficulty of similar discriminations may relate to the necessity to both strengthen rewarded representations and weaken overlap across similar representations. Key words: Arc; norepinephrine; odor discrimination; olfactory bulb; pattern separation; piriform cortex Significance Statement We show for the first time that adrenoceptors in anterior piriform cortex (aPC) must be engaged for adult rats to learn to discriminate highly similar odors.