Titin in Insect Spermatocyte Spindle Fibers Associates with Microtubules, Actin, Myosin and the Matrix Proteins Skeletor, Megator and Chromator
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Appropriate Roles of Cardiac Troponins in Evaluating Patients with Chest Pain
J Am Board Fam Pract: first published as 10.3122/jabfm.12.3.214 on 1 May 1999. Downloaded from MEDICAL PRACTICE Appropriate Roles of Cardiac Troponins in Evaluating Patients With Chest Pain Matthew S. Rice, MD, CPT, Me, USA, and David C. MacDonald, DO, Me, USA Background: Diagnosis of acute myocardial infarction relies upon the clinical history, interpretation of the electrocardiogram, and measurement of serum levels of cardiac enzymes. Newer biochemical markers of myocardial injury, such as cardiac troponin I and cardiac troponin T, are now being used instead of or along with the standard markers, the MB isoenzyme of creatine kinase (CK-MB) and lactate dehydrogenase. Methods: We performed a MEDLINE literature search (1987 to 1997) using the key words "troponin I," "troponin T," and "acute myocardial infarction." We reviewed selected articles related to the diagnostic and prognostic usefulness of these cardiac markers in evaluating patients with suspected myocardial infarction. Results: We found that (1) troponin I is a better cardiac marker than CK-MB for myocardial infarction because it is equally sensitive yet more specific for myocardial injury; (2) troponin T is a relatively poorer cardiac marker than CK-MB because it is less sensitive and less specific for myocardial injury; and (3) both troponin I and troponin T may be used as independent prognosticators of future cardiac events. Conclusions: Troponin I is a sensitive and specific marker for myocardial injury and can be used to predict the likelihood of future cardiac events. It is not much more expensive to measure than CK-MB. Over all, troponin I is a better cardiac marker than CK-MB and should become the preferred cardiac enzyme when evaluating patients with suspected myocardial infarction. -

Familial Adenomatous Polyposis Polymnia Galiatsatos, M.D., F.R.C.P.(C),1 and William D
American Journal of Gastroenterology ISSN 0002-9270 C 2006 by Am. Coll. of Gastroenterology doi: 10.1111/j.1572-0241.2006.00375.x Published by Blackwell Publishing CME Familial Adenomatous Polyposis Polymnia Galiatsatos, M.D., F.R.C.P.(C),1 and William D. Foulkes, M.B., Ph.D.2 1Division of Gastroenterology, Department of Medicine, The Sir Mortimer B. Davis Jewish General Hospital, McGill University, Montreal, Quebec, Canada, and 2Program in Cancer Genetics, Departments of Oncology and Human Genetics, McGill University, Montreal, Quebec, Canada Familial adenomatous polyposis (FAP) is an autosomal-dominant colorectal cancer syndrome, caused by a germline mutation in the adenomatous polyposis coli (APC) gene, on chromosome 5q21. It is characterized by hundreds of adenomatous colorectal polyps, with an almost inevitable progression to colorectal cancer at an average age of 35 to 40 yr. Associated features include upper gastrointestinal tract polyps, congenital hypertrophy of the retinal pigment epithelium, desmoid tumors, and other extracolonic malignancies. Gardner syndrome is more of a historical subdivision of FAP, characterized by osteomas, dental anomalies, epidermal cysts, and soft tissue tumors. Other specified variants include Turcot syndrome (associated with central nervous system malignancies) and hereditary desmoid disease. Several genotype–phenotype correlations have been observed. Attenuated FAP is a phenotypically distinct entity, presenting with fewer than 100 adenomas. Multiple colorectal adenomas can also be caused by mutations in the human MutY homologue (MYH) gene, in an autosomal recessive condition referred to as MYH associated polyposis (MAP). Endoscopic screening of FAP probands and relatives is advocated as early as the ages of 10–12 yr, with the objective of reducing the occurrence of colorectal cancer. -

Alpha-Actinin-3 R577X
Annals of Applied Sport Science, vol. 4, no. 4, pp. 01-06, Winter 2016 DOI: 10.18869/acadpub.aassjournal.4.4.1 Short Communication www.aassjournal.com www.AESAsport.com ISSN (Online): 2322 – 4479 Received: 20/03/2016 ISSN (Print): 2476–4981 Accepted: 10/06/2016 Alpha-actinin-3 R577X Polymorphism Profile of Turkish Professional Hip-Hop and Latin Dancers 1,2 * 1 1 2 1 1 Korkut Ulucan , Betul Biyik, Sezgin Kapici, Canan Sercan, Oznur Yilmaz, Tunc Catal 1Üsküdar Univerity, Haluk Turksoy Sok. No:14, Altunizade, Üsküdar, İstanbul, Turkey. 2Marmara University, BAsibuyuk Yolu 9/3 MAltepe Saglık Yerleşkesi, MAltepe, Istanbul, Turkey. ABSTRACT Actins are small globular filaments functioning in cell processes like muscle contraction, and stabilized to the sarcomeric Z- discs by actin binding proteins (actinins). One of the important gene coding for actin binding proteins in fast twitch fibers is alpha- actinin- 3 (ACTN3). In this research, we have conducted a gene profile study investigating the genotype and allele distributions of ACTN3 R577X polymorphism in Turkish professional hip- hop and latin dancers and compared them to non-dancers as a control group. 30 professional dancers and non-dancers were recruited for the study. A genotyping procedure was carried out by a newly introduced four-primer PCR methodology. For statistical analysis, the Chi-square test was used to compare data between the groups (p<0,05 evaluated as significant). Numbers and the percentages of dancers were 2 (7%), 21 (70%) and 7(23%) for RR, RX and XX genotypes, respectively. The same numbers and the percentages were 15 (50%), 8 (15%) and 7 (23%) for RR, RX and XX genotypes, respectively, for the controls. -

Troponin Variants in Congenital Myopathies: How They Affect Skeletal Muscle Mechanics
International Journal of Molecular Sciences Review Troponin Variants in Congenital Myopathies: How They Affect Skeletal Muscle Mechanics Martijn van de Locht , Tamara C. Borsboom, Josine M. Winter and Coen A. C. Ottenheijm * Department of Physiology, Amsterdam Cardiovascular Sciences, Amsterdam UMC, Location VUmc, 1081 HZ Amsterdam, The Netherlands; [email protected] (M.v.d.L.); [email protected] (T.C.B.); [email protected] (J.M.W.) * Correspondence: [email protected]; Tel.: +31-(0)-20-444-8123 Abstract: The troponin complex is a key regulator of muscle contraction. Multiple variants in skeletal troponin encoding genes result in congenital myopathies. TNNC2 has been implicated in a novel congenital myopathy, TNNI2 and TNNT3 in distal arthrogryposis (DA), and TNNT1 and TNNT3 in nemaline myopathy (NEM). Variants in skeletal troponin encoding genes compromise sarcomere function, e.g., by altering the Ca2+ sensitivity of force or by inducing atrophy. Several potential therapeutic strategies are available to counter the effects of variants, such as troponin activators, introduction of wild-type protein through AAV gene therapy, and myosin modulation to improve muscle contraction. The mechanisms underlying the pathophysiological effects of the variants in skeletal troponin encoding genes are incompletely understood. Furthermore, limited knowledge is available on the structure of skeletal troponin. This review focusses on the physiology of slow and fast skeletal troponin and the pathophysiology of reported variants in skeletal troponin encoding genes. A better understanding of the pathophysiological effects of these variants, together with enhanced knowledge regarding the structure of slow and fast skeletal troponin, will direct the development of Citation: van de Locht, M.; treatment strategies. -

Regulation of Titin-Based Cardiac Stiffness by Unfolded Domain Oxidation (Undox)
Regulation of titin-based cardiac stiffness by unfolded domain oxidation (UnDOx) Christine M. Loeschera,1, Martin Breitkreuzb,1, Yong Lia, Alexander Nickelc, Andreas Ungera, Alexander Dietld, Andreas Schmidte, Belal A. Mohamedf, Sebastian Kötterg, Joachim P. Schmitth, Marcus Krügere,i, Martina Krügerg, Karl Toischerf, Christoph Maackc, Lars I. Leichertj, Nazha Hamdanib, and Wolfgang A. Linkea,2 aInstitute of Physiology II, University of Munster, 48149 Munster, Germany; bInstitute of Physiology, Ruhr University Bochum, 44801 Bochum, Germany; cComprehensive Heart Failure Center Wuerzburg, University Clinic Wuerzburg, 97078 Wuerzburg, Germany; dDepartment of Internal Medicine II, University Hospital Regensburg, 93053 Regensburg, Germany; eInstitute for Genetics, University of Cologne, 50931 Cologne, Germany; fDepartment of Cardiology and Pneumology, University Medicine Goettingen, 37075 Goettingen, Germany; gDepartment of Cardiovascular Physiology, Heinrich Heine University, 40225 Düsseldorf, Germany; hDepartment of Pharmacology and Clinical Pharmacology, Heinrich Heine University, 40225 Düsseldorf, Germany; iCenter for Molecular Medicine and Excellence Cluster "Cellular Stress Responses in Aging-Associated Diseases" (CECAD), University of Cologne, 50931 Cologne, Germany; and jInstitute for Biochemistry and Pathobiochemistry, Ruhr University Bochum, 44801 Bochum, Germany Edited by Jonathan Seidman, Harvard University, Boston, MA, and approved August 12, 2020 (received for review March 14, 2020) The relationship between oxidative stress and -

Actin-Troponin-Tropomyosin Complex (Muscle Relaxation/Cooperativity/Regulated Actin) Lois E
Proc. Nati. Acad. Sci. USA Vol. 77, No. 5, pp. 2616-2620, May 1980 Biochemistry Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex (muscle relaxation/cooperativity/regulated actin) Lois E. GREENE AND EVAN EISENBERG Laboratory of Cell Biology, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, Maryland 20205 Communicated by Terrell L. Hill, February 22, 1980 ABSTRACT The binding of myosin subfragment-1 (S-i) to of a few S-1 molecules, free of ATP, to the actin filament and the F-actin-troponin-tropomyosin complex (regulated F-actin). pushing the tropomyosin away from its inhibitory position, thus was examined in the presence of ADP (ionic strength, 0.23 M; preventing inhibition of the ATPase activity even in the absence 220C) by using the ultracentrifuge and S-1 blocked at SHI with iodo["4C]acetamide. S-1ADP binds with positive cooperativity of Ca2+. Cooperative responses have also been observed in the to regulated F-actin, both in the presence and absence of cal- presence of Ca2+. Weber and coworkers (6) found that at high cium; it binds independently to unregulated actin. With and S-1 concentration the ATPase activity of regulated acto-S-1 can without CaO+ at very low levels of occupancy of the regulated be potentiated so that it is higher than the ATPase activity of actin by S-19ADP, S-1*ADP binds to the regulated actin with acto*S-1 in the absence of troponin-tropomyosin. <1% of the strength that it binds to unregulated actin, whereas The cooperative responses observed with regulated actin are at high levels of occupancy of the regulated actin by S-1-ADP, S-1ADP binds about 3-fold more strongly to the regulated actin fundamental to our understanding of the biochemical basis of than it does to unregulated actin. -

Postmortem Changes in the Myofibrillar and Other Cytoskeletal Proteins in Muscle
BIOCHEMISTRY - IMPACT ON MEAT TENDERNESS Postmortem Changes in the Myofibrillar and Other C'oskeletal Proteins in Muscle RICHARD M. ROBSON*, ELISABETH HUFF-LONERGAN', FREDERICK C. PARRISH, JR., CHIUNG-YING HO, MARVIN H. STROMER, TED W. HUIATT, ROBERT M. BELLIN and SUZANNE W. SERNETT introduction filaments (titin), and integral Z-line region (a-actinin, Cap Z), as well as proteins of the intermediate filaments (desmin, The cytoskeleton of "typical" vertebrate cells contains paranemin, and synemin), Z-line periphery (filamin) and three protein filament systems, namely the -7-nm diameter costameres underlying the cell membrane (filamin, actin-containing microfilaments, the -1 0-nm diameter in- dystrophin, talin, and vinculin) are listed along with an esti- termediate filaments (IFs), and the -23-nm diameter tubu- mate of their abundance, approximate molecular weights, lin-containing microtubules (Robson, 1989, 1995; Robson and number of subunits per molecule. Because the myofibrils et al., 1991 ).The contractile myofibrils, which are by far the are the overwhelming components of the skeletal muscle cell major components of developed skeletal muscle cells and cytoskeleton, the approximate percentages of the cytoskel- are responsible for most of the desirable qualities of muscle eton listed for the myofibrillar proteins (e.g., myosin, actin, foods (Robson et al., 1981,1984, 1991 1, can be considered tropomyosin, a-actinin, etc.) also would represent their ap- the highly expanded corollary of the microfilament system proximate percentages of total myofibrillar protein. of non-muscle cells. The myofibrils, IFs, cell membrane skel- eton (complex protein-lattice subjacent to the sarcolemma), Some Important Characteristics, Possible and attachment sites connecting these elements will be con- Roles, and Postmortem Changes of Key sidered as comprising the muscle cell cytoskeleton in this Cytoskeletal Proteins review. -

Α-Actinin/Titin Interaction: a Dynamic and Mechanically Stable Cluster of Bonds in the Muscle Z-Disk
α-Actinin/titin interaction: A dynamic and mechanically stable cluster of bonds in the muscle Z-disk Marco Grisona, Ulrich Merkela, Julius Kostanb, Kristina Djinovic-Carugob,c, and Matthias Riefa,d,1 aPhysik Department E22, Technische Universität München, 85748 Garching, Germany; bDepartment of Structural and Computational Biology, Max F. Perutz Laboratories, University of Vienna, A-1030 Vienna, Austria; cDepartment of Biochemistry, Faculty of Chemistry and Chemical Technology, University of Ljubljana, SI-1000 Ljubljana, Slovenia; and dMunich Center for Integrated Protein Science, 81377 Munich, Germany Edited by James A. Spudich, Stanford University School of Medicine, Stanford, CA, and approved December 16, 2016 (received for review August 2, 2016) Stable anchoring of titin within the muscle Z-disk is essential for In humans, the isoforms of titin exhibit four to seven Z-repeats preserving muscle integrity during passive stretching. One of the (15, 16, 20). The structure of the EF3-4 hands complex with titin main candidates for anchoring titin in the Z-disk is the actin cross- Z-repeat 7 shows the bound Z-repeat in an α-helical confor- linker α-actinin. The calmodulin-like domain of α-actinin binds to mation (21). In solution assays, binding affinities of various the Z-repeats of titin. However, the mechanical and kinetic prop- Z-repeats to EF3-4 were determined to lie in the micromolar erties of this important interaction are still unknown. Here, we use range (22). Micromolar affinity points only to a moderately a dual-beam optical tweezers assay to study the mechanics of this stable interaction, the kinetics of which are unknown. -
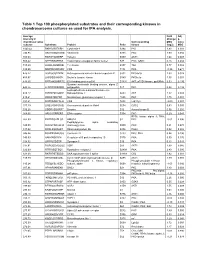
Table 1 Top 100 Phosphorylated Substrates and Their Corresponding Kinases in Chondrosarcoma Cultures As Used for IPA Analysis
Table 1 Top 100 phosphorylated substrates and their corresponding kinases in chondrosarcoma cultures as used for IPA analysis. Average Fold Adj intensity in Change p- chondrosarcoma Corresponding MSC value cultures Substrate Protein Psite kinase (log2) MSC 1043.42 RKKKVSSTKRH Cytohesin-1 S394 PKC 1.83 0.001 746.95 RKGYRSQRGHS Vitronectin S381 PKC 1.00 0.056 709.03 RARSTSLNERP Tuberin S939 AKT1 1.64 0.008 559.42 SPPRSSLRRSS Transcription elongation factor A-like1 S37 PKC; GSK3 0.18 0.684 515.29 LRRSLSRSMSQ Telethonin S157 Titin 0.77 0.082 510.00 MQPDNSSDSDY CD5 T434 PKA -0.35 0.671 476.27 GGRGGSRARNL Heterogeneous nuclear ribonucleoprotein K S302 PKCdelta 1.03 0.028 455.97 LKPGSSHRKTK Bruton's tyrosine kinase S180 PKCbeta 1.55 0.001 444.65 RRRMASMQRTG E1A binding protein p300 S1834 AKT; p70S6 kinase; pp90Rsk 0.53 0.195 Guanine nucleotide binding protein, alpha Z 440.26 HLRSESQRQRR polypeptide S27 PKC 0.88 0.199 6-phosphofructo-2-kinase/fructose-2,6- 424.12 RPRNYSVGSRP biphosphatase 2 S483 AKT 1.32 0.003 419.61 KKKIATRKPRF Metabotropic glutamate receptor 1 T695 PKC 1.75 0.001 391.21 DNSSDSDYDLH CD5 T453 Lck; Fyn -2.09 0.001 377.39 LRQLRSPRRAQ Ras associated protein Rab4 S204 CDC2 0.63 0.091 376.28 SSQRVSSYRRT Desmin S12 Aurora kinase B 0.56 0.255 369.05 ARIGGSRRERS EP4 receptor S354 PKC 0.29 0.543 RPS6 kinase alpha 3; PKA; 367.99 EPKRRSARLSA HMG14 S7 PKC -0.01 0.996 Peptidylglycine alpha amidating 349.08 SRKGYSRKGFD monooxygenase S930 PKC 0.21 0.678 347.92 RRRLSSLRAST Ribosomal protein S6 S236 PAK2 0.02 0.985 346.84 RSNPPSRKGSG Connexin -

Current Understanding of the Role of Cytoskeletal Cross-Linkers in the Onset and Development of Cardiomyopathies
International Journal of Molecular Sciences Review Current Understanding of the Role of Cytoskeletal Cross-Linkers in the Onset and Development of Cardiomyopathies Ilaria Pecorari 1, Luisa Mestroni 2 and Orfeo Sbaizero 1,* 1 Department of Engineering and Architecture, University of Trieste, 34127 Trieste, Italy; [email protected] 2 University of Colorado Cardiovascular Institute, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA; [email protected] * Correspondence: [email protected]; Tel.: +39-040-5583770 Received: 15 July 2020; Accepted: 10 August 2020; Published: 15 August 2020 Abstract: Cardiomyopathies affect individuals worldwide, without regard to age, sex and ethnicity and are associated with significant morbidity and mortality. Inherited cardiomyopathies account for a relevant part of these conditions. Although progresses have been made over the years, early diagnosis and curative therapies are still challenging. Understanding the events occurring in normal and diseased cardiac cells is crucial, as they are important determinants of overall heart function. Besides chemical and molecular events, there are also structural and mechanical phenomena that require to be investigated. Cell structure and mechanics largely depend from the cytoskeleton, which is composed by filamentous proteins that can be cross-linked via accessory proteins. Alpha-actinin 2 (ACTN2), filamin C (FLNC) and dystrophin are three major actin cross-linkers that extensively contribute to the regulation of cell structure and mechanics. Hereby, we review the current understanding of the roles played by ACTN2, FLNC and dystrophin in the onset and progress of inherited cardiomyopathies. With our work, we aim to set the stage for new approaches to study the cardiomyopathies, which might reveal new therapeutic targets and broaden the panel of genes to be screened. -

Association of Titin and Myosin Heavy Chain in Developing Skeletal Muscle (Myogenesis/Cytoskeleton/Assembly in Vvo) W
Proc. Natl. Acad. Sci. USA Vol. 89, pp. 74%-7500, August 1992 Cell Biology Association of titin and myosin heavy chain in developing skeletal muscle (myogenesis/cytoskeleton/assembly in vvo) W. B. ISAACS*, I. S. KIM, A. STRUVE, AND A. B. FULTONt Department of Biochemistry, University of Iowa, Iowa City, IA 52242 Communicated by Sheldon Penman, May 22, 1992 ABSTRACT To understand molecular interactions that deficient medium (10). After labeling, cultures either were organize developing myoflbrils, we examined the biosynthesis extracted immediately or were chased by adding complete and interaction of titin and myosin heavy chain in cultures of medium supplemented with 2 mM unlabeled methionine and developing muscle. Use of pulse-labeling, immunoprecipita- incubating at 370C for various times before extraction. Ex- tion, and a reversible cross-linking procedure demonstrates tractions used 0.5% Triton X-100 in extraction buffer (100 that within minutes of synthesis, titin and myosin heavy chain mM KCI/10 mM Pipes, pH 6.8/300 mM sucrose/2 mM can be chemically cross-linked into very large, detergent- MgCI2/1 mM EGTA) containing protease inhibitors (1 mM resistant complexes retaining many features of intact myo- phenylmethylsulfonyl fluoride and 100 mM leupeptin; ref. tubes. These complexes, predominantly of titin and myosin, 11). occur very early in myofibrillogenesis as well as later. These Immunoprecipitation. Titin was precipitated by using a data suggest that synthesis and assembly oftitin and myosin are mouse monoclonal antibody (mAb), AMF-1, as described temporally and spatially coordinated in nascent myofibrils and (10). Muscle-specific myosin heavy chain (hereafter myosin) support the hypothesis that titin molecules help to organize was precipitated with mAb MF-20 (12), a gift ofD. -

Titin N2A Domain and Its Interactions at the Sarcomere
International Journal of Molecular Sciences Review Titin N2A Domain and Its Interactions at the Sarcomere Adeleye O. Adewale and Young-Hoon Ahn * Department of Chemistry, Wayne State University, Detroit, MI 48202, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-(313)-577-1384 Abstract: Titin is a giant protein in the sarcomere that plays an essential role in muscle contraction with actin and myosin filaments. However, its utility goes beyond mechanical functions, extending to versatile and complex roles in sarcomere organization and maintenance, passive force, mechanosens- ing, and signaling. Titin’s multiple functions are in part attributed to its large size and modular structures that interact with a myriad of protein partners. Among titin’s domains, the N2A element is one of titin’s unique segments that contributes to titin’s functions in compliance, contraction, structural stability, and signaling via protein–protein interactions with actin filament, chaperones, stress-sensing proteins, and proteases. Considering the significance of N2A, this review highlights structural conformations of N2A, its predisposition for protein–protein interactions, and its multiple interacting protein partners that allow the modulation of titin’s biological effects. Lastly, the nature of N2A for interactions with chaperones and proteases is included, presenting it as an important node that impacts titin’s structural and functional integrity. Keywords: titin; N2A domain; protein–protein interaction 1. Introduction Citation: Adewale, A.O.; Ahn, Y.-H. The complexity of striated muscle is defined by the intricate organization of its com- Titin N2A Domain and Its ponents [1]. The involuntary cardiac and voluntary skeletal muscles are the primary types Interactions at the Sarcomere.