University of Groningen Ineffective Frankia in Wet Alder Soils Wolters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogénie Et Évolution Du Genre Frankia Imen Nouioui
Phylogénie et évolution du genre Frankia Imen Nouioui To cite this version: Imen Nouioui. Phylogénie et évolution du genre Frankia. Biologie végétale. Université Claude Bernard - Lyon I, 2014. Français. NNT : 2014LYO10087. tel-01089349 HAL Id: tel-01089349 https://tel.archives-ouvertes.fr/tel-01089349 Submitted on 1 Dec 2014 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. N° d’ordre 87 – 2014 Année 2014 THÈSE DE L’UNIVERSITE DE LYON Délivrée par L’UNIVERSITE CLAUDE BERNARD LYON 1 ECOLE DOCTORALE : Evolution Ecosystèmes Microbiologie Modélisation DIPLOME DE DOCTORAT (arrêté du 7 Août 2006) Soutenue publiquement le 23 juin 2014 par Melle Imen NOUIOUI Phylogénie et Évolution du genre Frankia Directeurs de thèse : Maria P. FERNANDEZ & Maher GTARI Jury : M. Abdellatif Boudabous Professeur à l’Université Tunis El Manar Président M. Ridha Mhamdi Professeur au Centre de Biotechnologie de Borj Cédria Rapporteur M. Sergio Svistoonoff Chargé de recherche à l’IRD, Montpellier Rapporteur M. Maher Gtari Professeur à l’Université de Carthage Examinateur Mme. Maria -

Global Survey of Ex Situ Betulaceae Collections Global Survey of Ex Situ Betulaceae Collections
Global Survey of Ex situ Betulaceae Collections Global Survey of Ex situ Betulaceae Collections By Emily Beech, Kirsty Shaw and Meirion Jones June 2015 Recommended citation: Beech, E., Shaw, K., & Jones, M. 2015. Global Survey of Ex situ Betulaceae Collections. BGCI. Acknowledgements BGCI gratefully acknowledges the many botanic gardens around the world that have contributed data to this survey (a full list of contributing gardens is provided in Annex 2). BGCI would also like to acknowledge the assistance of the following organisations in the promotion of the survey and the collection of data, including the Royal Botanic Gardens Edinburgh, Yorkshire Arboretum, University of Liverpool Ness Botanic Gardens, and Stone Lane Gardens & Arboretum (U.K.), and the Morton Arboretum (U.S.A). We would also like to thank contributors to The Red List of Betulaceae, which was a precursor to this ex situ survey. BOTANIC GARDENS CONSERVATION INTERNATIONAL (BGCI) BGCI is a membership organization linking botanic gardens is over 100 countries in a shared commitment to biodiversity conservation, sustainable use and environmental education. BGCI aims to mobilize botanic gardens and work with partners to secure plant diversity for the well-being of people and the planet. BGCI provides the Secretariat for the IUCN/SSC Global Tree Specialist Group. www.bgci.org FAUNA & FLORA INTERNATIONAL (FFI) FFI, founded in 1903 and the world’s oldest international conservation organization, acts to conserve threatened species and ecosystems worldwide, choosing solutions that are sustainable, based on sound science and take account of human needs. www.fauna-flora.org GLOBAL TREES CAMPAIGN (GTC) GTC is undertaken through a partnership between BGCI and FFI, working with a wide range of other organisations around the world, to save the world’s most threated trees and the habitats which they grow through the provision of information, delivery of conservation action and support for sustainable use. -

Biosystematics and Phenology of Alnus Maritima (Betulaceae) James Alan Schrader Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2002 Biosystematics and phenology of Alnus maritima (Betulaceae) James Alan Schrader Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Botany Commons, and the Ecology and Evolutionary Biology Commons Recommended Citation Schrader, James Alan, "Biosystematics and phenology of Alnus maritima (Betulaceae) " (2002). Retrospective Theses and Dissertations. 1028. https://lib.dr.iastate.edu/rtd/1028 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of thi* reproduction Is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. -

Supplementary Material
Xiang et al., Page S1 Supporting Information Fig. S1. Examples of the diversity of diaspore shapes in Fagales. Fig. S2. Cladogram of Fagales obtained from the 5-marker data set. Fig. S3. Chronogram of Fagales obtained from analysis of the 5-marker data set in BEAST. Fig. S4. Time scale of major fagalean divergence events during the past 105 Ma. Fig. S5. Confidence intervals of expected clade diversity (log scale) according to age of stem group. Fig. S6. Evolution of diaspores types in Fagales with BiSSE model. Fig. S7. Evolution of diaspores types in Fagales with Mk1 model. Fig. S8. Evolution of dispersal modes in Fagales with MuSSE model. Fig. S9. Evolution of dispersal modes in Fagales with Mk1 model. Fig. S10. Reconstruction of pollination syndromes in Fagales with BiSSE model. Fig. S11. Reconstruction of pollination syndromes in Fagales with Mk1 model. Fig. S12. Reconstruction of habitat shifts in Fagales with MuSSE model. Fig. S13. Reconstruction of habitat shifts in Fagales with Mk1 model. Fig. S14. Stratigraphy of fossil fagalean genera. Table S1 Genera of Fagales indicating the number of recognized and sampled species, nut sizes, habits, pollination modes, and geographic distributions. Table S2 List of taxa included in this study, sources of plant material, and GenBank accession numbers. Table S3 Primers used for amplification and sequencing in this study. Table S4 Fossil age constraints utilized in this study of Fagales diversification. Table S5 Fossil fruits reviewed in this study. Xiang et al., Page S2 Table S6 Statistics from the analyses of the various data sets. Table S7 Estimated ages for all families and genera of Fagales using BEAST. -
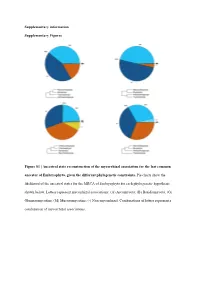
Ancestral State Reconstruction of the Mycorrhizal Association for the Last Common Ancestor of Embryophyta, Given the Different Phylogenetic Constraints
Supplementary information Supplementary Figures Figure S1 | Ancestral state reconstruction of the mycorrhizal association for the last common ancestor of Embryophyta, given the different phylogenetic constraints. Pie charts show the likelihood of the ancestral states for the MRCA of Embryophyta for each phylogenetic hypothesis shown below. Letters represent mycorrhizal associations: (A) Ascomycota; (B) Basidiomycota; (G) Glomeromycotina; (M) Mucoromycotina; (-) Non-mycorrhizal. Combinations of letters represent a combination of mycorrhizal associations. Austrocedrus chilensis Chamaecyparis obtusa Sequoiadendron giganteum Prumnopitys taxifolia Prumnopitys Prumnopitys montana Prumnopitys Prumnopitys ferruginea Prumnopitys Araucaria angustifolia Araucaria Dacrycarpus dacrydioides Dacrycarpus Taxus baccata Podocarpus oleifolius Podocarpus Afrocarpus falcatus Afrocarpus Ephedra fragilis Nymphaea alba Nymphaea Gnetum gnemon Abies alba Abies balsamea Austrobaileya scandens Austrobaileya Abies nordmanniana Thalictrum minus Thalictrum Abies homolepis Caltha palustris Caltha Abies magnifica ia repens Ranunculus Abies religiosa Ranunculus montanus Ranunculus Clematis vitalba Clematis Keteleeria davidiana Anemone patens Anemone Tsuga canadensis Vitis vinifera Vitis Tsuga mertensiana Saxifraga oppositifolia Saxifraga Larix decidua Hypericum maculatum Hypericum Larix gmelinii Phyllanthus calycinus Phyllanthus Larix kaempferi Hieronyma oblonga Hieronyma Pseudotsuga menziesii Salix reinii Salix Picea abies Salix polaris Salix Picea crassifolia Salix herbacea -

Tree Pollens
Tree pollens Allergy – Which allergens? Author: Dr Harris Steinman, Allergy Resources International, P O Box 565, Milnerton 7435, South Africa, [email protected]. All rights reserved. No part of this publication may be reproduced in any form without the written consent of Phadia AB. ©Phadia AB, 2008 Design: RAK Design AB, 2008 Printed by: Åtta.45 Tryckeri AB, Solna, Sweden ISBN 91-973440-5-2 Contents Introduction .........................................................................5 t19 Acacia (Acacia longifolia) .........................................11 t5 American beech (Fagus grandifolia) ...........................14 t73 Australian pine (Casuarina equisetifolia) ....................17 t37 Bald cypress (Taxodium distichum) ...........................20 t56 Bayberry (Myrica cerifera) .........................................22 t1 Box-elder (Acer negundo) .........................................24 t212 Cedar (Libocedrus decurrens) ...................................27 t45 Cedar elm (Ulmus crassifolia) ...................................29 t206 Chestnut (Castanea sativa) .......................................32 t3 Common silver birch (Betula verrucosa) .....................35 t14 Cottonwood (Populus deltoides) ................................45 t222 Cypress (Cupressus arizonica) ...................................49 t214 Date (Phoenix canariensis) .......................................56 t207 Douglas fir (Pseudotsuga taxifolia) .............................59 t205 Elder (Sambucus nigra) ............................................60 -

Systematics of Alnus Maritima (Seaside Alder) Resolved by ISSR Polymorphisms and Morphological Characters
J. AMER. SOC. HORT. SCI. 129(2):231–236. 2004. Systematics of Alnus maritima (Seaside Alder) Resolved by ISSR Polymorphisms and Morphological Characters James A. Schrader1 and William R. Graves Department of Horticulture, Iowa State University, Ames, IA 50011-1100 ADDITIONAL INDEX WORDS. subg. Clethropsis, subspp. oklahomensis and georgiensis, molecular phylogeny, morphometrics, cladistics, inter-simple sequence repeats, fl uorescent primers, simultaneous analysis, ABI PRISM, GeneScan ABSTRACT. Alnus maritima (Marsh.) Muhl. ex Nutt. is a rare woody plant species that exists as three subspecies found in widely disjunct locations in the United States. Although there is a growing interest in the phytogeography, ecology, conservation, and landscape potential of this species, the phylogeny of A. maritima has not yet been resolved by using molecular methods. We have combined a relatively new method of genome fi ngerprinting, ISSR-PCR, and the auto- mated imaging capabilities of GeneScan technology to investigate the molecular systematics of A. maritima. Based on the molecular evidence from 108 ISSR loci, we confi rm that the three disjunct populations of A. maritima have diverged suffi ciently to be classifi ed as subspecies. Our molecular phylogeny of the three subspecies of A. maritima agreed in topology with a phylogeny produced from morphological data and showed that subsp. oklahomensis is the most distinct of the three subspecies and was the fi rst to diverge. The simultaneous analysis of molecular and morphological data provides a detailed and balanced phylogeny reconstruction for the three subspecies. Our results support the theory that A. maritima originated in Asia, migrated into North America across the Bering land bridge, and was established over a large range in the New World before being forced into its present meager disjunct distribution. -
The Nitrogen-Fixing Frankia Significantly Increases Growth, Uprooting Resistance and Root Tensile Strength of Alnus Formosana
Vol. 17(7), pp. 213-225, 14 February, 2018 DOI: 10.5897/AJB2017.16289 Article Number: 9CFD65F55921 ISSN 1684-5315 African Journal of Biotechnology Copyright © 2018 Author(s) retain the copyright of this article http://www.academicjournals.org/AJB Full Length Research Paper The nitrogen-fixing Frankia significantly increases growth, uprooting resistance and root tensile strength of Alnus formosana Jung-Tai Lee* and Sung-Ming Tsai Department of Forestry and Natural Resources, College of Agriculture, National Chiayi University, Taiwan. Received 19 October, 2017; Accepted 18 January, 2018 Restoration of Alnus formosana (Burk.) Makino on landslide areas is important for agroforestry, forestry and soil erosion control in Taiwan. To ensure successful reforestation, A. formosana seedlings have to develop strong root system for nutrient and water acquisition as well as anchorage. Inoculating of A. formosana with symbiotic nitrogen-fixing actinobacteria Frankia may help mitigate drought and nutrient deficiencies on landslide sites. However, the effects of Frankia inoculation on growth, root architecture and mechanical properties of A. formosana seedlings are not well understood. In this research, a Frankia strain AF1 was isolated from actinorhizal nodules of local A. formosana and recognized as Frankia species, and its influences on growth performance and root mechanical properties of A. formosana seedlings were examined and analyzed. The results showed that the inoculated seedlings had significantly larger height and root biomass, longer root length, and more root tip number than that of the non-inoculated controls. Consistently, the inoculated seedlings had statistically significant higher uprooting resistance, root tensile resistance force and tensile strength than the controls. The results reveal that this native Frankia strain can promote growth performance, root system architecture, anchorage ability and root tensile strength of A. -

THE ACTINORHIZAL SYMBIOSIS of the EARLIEST DIVERGENT FRANKIA CLUSTER Nguyen Thi Thanh Van
THE ACTINORHIZAL SYMBIOSIS OF THE EARLIEST DIVERGENT FRANKIA CLUSTER Nguyen Thi Thanh Van The actinorhizal symbiosis of the earliest divergent Frankia cluster Nguyen Thi Thanh Van ©Nguyen Thi Thanh Van, Stockholm University 2017 ISBN print 978-91-7649-716-6 ISBN PDF 978-91-7649-717-3 Printed in Sweden by US-AB, Stockholm 2017 Distributor: Department of Ecology, Environment and Plant Sciences Stockholm University, Stockholm, Sweden Con kính tặng Ba Sammanfattning Under de senaste åren har allt större tonvikt lagts vid vikten av att utveckla en världsomspännande utvecklingsmässigt ekologiskt hållbar jordbruksnäring. Nödvändigheten av att minska beroendet av syntetiska kvävebaserade gödningsmedel har lett till ett alltmer växande forskningsfält baserat på vikten av biologisk kvävefixering, med emfas på rotknölsymbios. Mina studier har fokuserat på mekanismerna i och med actinorhizalsymbios, i.e den symbiotiska interaktionen mellan olika kvävefixerande actinobacteria -genus Frankia. i förhållande till en mycket skiftande grupp av växter från åtta olika växtfamiljer, som sammantaget kallas actinorhizalväxter. Frankia kluster II har visats vara en systerklad i förhållande till alla andra kluster, vilket gör att denna speciella gren kan ge insikt i hur Frankia kluster II kan tillföra värdefull information om den evolutionära bakgrunden i och med actinorhizalsymbios. Det första fullt sekvenserade genomet av en medlem från detta kluster, Candidatus Frankia datiscae Dg1 med ursprung från Pakistan, visar att genomet infattar nod generna nodABC som svarar för framställandet av lipochitooligosaccharid Nod-faktorer, vilket särskiljer denna medlem från andra grupper av Frankia som saknar dessa gener. Arbetet i och med denna avhandling är grundat på gDNA isolerat från sex inokula, tre från Nordamerika (USA; två från Kalifornien och ett från Alaska), ett från Europa (Frankrike), ett från Asien (Japan) och ett från Oceanien (Papua Nya Guinea). -

Nodulation and Growth of Alnus Nitida and Alnus Maritima Inoculated with Species-Specifi C and Nonspecifi C Frankia1
This Journal of Environmental Horticulture article is reproduced with the consent of the Horticultural Research Institute (HRI – www.hriresearch.org), which was established in 1962 as the research and development affiliate of the American Nursery & Landscape Association (ANLA – http://www.anla.org). HRI’s Mission: To direct, fund, promote and communicate horticultural research, which increases the quality and value of ornamental plants, improves the productivity and profitability of the nursery and landscape industry, and protects and enhances the environment. The use of any trade name in this article does not imply an endorsement of the equipment, product or process named, nor any criticism of any similar products that are not mentioned. Copyright, All Rights Reserved Nodulation and Growth of Alnus nitida and Alnus maritima Inoculated with Species-specifi c and Nonspecifi c Frankia1 James A. Schrader and William R. Graves2 Department of Horticulture, Iowa State University, Ames, IA 50011-1100 Abstract Actinorhizal plants form N2-fi xing symbioses with soil-borne bacteria of the genus Frankia. Potential exists for development of sustainable, actinorhizal nursery crops that obtain most of their required N through N2 fi xation, but information on host-symbiont specifi city, presence of compatible Frankia in soils, and techniques to inoculate during plant production is lacking. Our objectives were to determine the effect of inoculum type and source and the effect of supplemental N on nodulation, growth, and N content of two actinorhizal species, Alnus nitida (Spach) Endl. and Alnus maritima (Marsh.) Muhl. ex Nutt. Plants of both species were subjected to one of four inoculum treatments (two crushed-nodule inocula: species-specifi c and cross inoculation, and two soil inocula: soil collected beneath native Alnus rubra Bong. -

AUTUMN FLOWERING ALDER TREES Dr Linda Newstrom-Lloyd (Trees for Bees Botanist), and Dr Angus Mcpherson (Trees for Bees Farm Planting Adviser)
34 NEW ZEALAND BEEKEEPER, APRIL 2020 TREES FOR BEES CORNER STAR PERFORMERS PART 11: AUTUMN FLOWERING ALDER TREES Dr Linda Newstrom-Lloyd (Trees for Bees Botanist), and Dr Angus McPherson (Trees for Bees Farm Planting Adviser) Trees for Bees has produced a series of fact sheets showcasing the ‘best of the best’ bee plants that will maximise nutritional benefits for your bees. In this issue of the journal, the team explains why the autumn-flowering alders are ‘star performers’. For more information, see www.treesforbeesnz.org. Four species of alder trees regularly flower Leopold et al., 2012). Most alder species flower in autumn and provide excellent pollen to Autumn is a critical in spring but four of them regularly flower strengthen bee colonies for wintertime. time for bee in autumn: Alnus nitida, A. formosana and A. nepalensis, which are native to Asia, and A. The autumn-flowering alders (Alnus spp.) nutrition because maritima, native to North America. These four are important star performers because they without good pollen autumn-flowering alders are not common provide plentiful pollen when bee colonies for protein and in New Zealand nurseries, but Appletons has are preparing for winter. The diversity of often had some of them in stock. pollen and nectar sources in autumn is very nectar for energy low compared to spring and summer. Our at this time of Flowers working list of candidate bee plants has only about 15–20 native species and a similar year, the number Alder trees are deciduous and wind number of exotic species that we are planting of new winter pollinated, which is typical of trees in the birch family. -

Species Identification of Alnus (Betulaceae) Using Nrdna and Cpdna Genetic Markers
Molecular Ecology Resources (2010) 10, 594–605 doi: 10.1111/j.1755-0998.2009.02815.x DNA BARCODING Species identification of Alnus (Betulaceae) using nrDNA and cpDNA genetic markers BAO-QING REN,*† XIAO-GUO XIANG*† and ZHI-DUAN CHEN* *State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Xiangshan, Beijing 100093, China, †Graduate School of the Chinese Academy of Sciences, Beijing 100039, China Abstract One nuclear and three chloroplast DNA regions (ITS, rbcL, matK and trnH-psbA)wereused to identify the species of Alnus (Betulaceae). The results showed that 23 out of all 26 Alnus species in the world, represented by 131 samples, had their own specific molecular character states, especially for three morphologically confused species (Alnus formosana, Alnus japon- ica and Alnus maritima). The discriminating power of the four markers at the species level was 10% (rbcL), 31.25% (matK), 63.6% (trnH-psbA) and 76.9% (ITS). For ITS, the mean value of genetic distance between species was more than 10 times the intraspecific distance (0.009%), and 13 species had unique character states that differentiated them from other spe- cies of Alnus.ThetrnH-psbA region had higher mean values of genetic distance between and within species (2.1% and 0.68% respectively) than any other region tested. Using the trnH- psbA region, 13 species are distinguished from 22 species, and seven species have a single diagnostic site. The combination of two regions, ITS and trnH-psbA, is the best choice for DNA identification of Alnus species, as an improvement and supplement for morphologically based taxonomy.