(DEA) Placed the Oripavine Into Schedule I1 of the Controlled Su
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Alternative Formats If You Require This Document in an Alternative Format, Please Contact: [email protected]
University of Bath PHD The extraction and chemistry of the metabolites of Mimosa tenuiflora and Papaver somniferum Ninan, Aleyamma Award date: 1990 Awarding institution: University of Bath Link to publication Alternative formats If you require this document in an alternative format, please contact: [email protected] General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 23. Sep. 2021 THE EXTRACTION AND CHEMISTRY OF THE METABOLITES OF MIMOSA TENUIFLORA AND PAP AVER SOMNIFERUM. submitted by ALEYAMMA NINAN for the degree of Doctor of Philosophy of the University of Bath 1990 Attention is drawn to the fact that the copyright of this thesis rests with its author. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with its author and that no quotation from the thesis and no information derived from it may be published without prior consent of the author. -

ECO-Ssls for Pahs
Ecological Soil Screening Levels for Polycyclic Aromatic Hydrocarbons (PAHs) Interim Final OSWER Directive 9285.7-78 U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response 1200 Pennsylvania Avenue, N.W. Washington, DC 20460 June 2007 This page intentionally left blank TABLE OF CONTENTS 1.0 INTRODUCTION .......................................................1 2.0 SUMMARY OF ECO-SSLs FOR PAHs......................................1 3.0 ECO-SSL FOR TERRESTRIAL PLANTS....................................4 5.0 ECO-SSL FOR AVIAN WILDLIFE.........................................8 6.0 ECO-SSL FOR MAMMALIAN WILDLIFE..................................8 6.1 Mammalian TRV ...................................................8 6.2 Estimation of Dose and Calculation of the Eco-SSL ........................9 7.0 REFERENCES .........................................................16 7.1 General PAH References ............................................16 7.2 References Used for Derivation of Plant and Soil Invertebrate Eco-SSLs ......17 7.3 References Rejected for Use in Derivation of Plant and Soil Invertebrate Eco-SSLs ...............................................................18 7.4 References Used in Derivation of Wildlife TRVs .........................25 7.5 References Rejected for Use in Derivation of Wildlife TRV ................28 i LIST OF TABLES Table 2.1 PAH Eco-SSLs (mg/kg dry weight in soil) ..............................4 Table 3.1 Plant Toxicity Data - PAHs ..........................................5 Table 4.1 -

Report of the International Narcotics Control Board for 2010
Report of the International Narcotics Control Board involving treatment for cocaine abuse accounted for 510. According to the 2009 AIDS Epidemic Update, 65 per cent of all cases involving treatment for published by the Joint United Nations Programme on substance abuse in 1998, and that figure decreased, in HIV/AIDS and WHO, an estimated 29 per cent of the relative terms, to 49 per cent in 2008. For the past more than 2 million Latin Americans who abuse drugs 10 years, cocaine has been the primary drug of abuse by injection are infected with HIV. HIV epidemics among persons treated for drug problems in the region. among such drug abusers in the region tend to be concentrated in the Southern Cone. It is estimated that 506. Demand for “crack” cocaine appears to be in Argentina alone, almost half of the persons who emerging in some countries in South America. In 2008, abuse drugs by injection are infected with HIV. seizures of “crack” cocaine were reported in Argentina, Brazil, Chile, Paraguay and Venezuela (Bolivarian Republic of). In the Bolivarian Republic of Venezuela, C. Asia lifetime prevalence of the abuse of “crack” cocaine among the population aged 15-70 is 11.9 per cent. In East and South-East Asia that country, about a quarter of the persons who received treatment for drug addiction were addicted to 1. Major developments “crack” cocaine. In 2010, the Government of Brazil launched its integrated plan to combat “crack” cocaine 511. In East and South-East Asia, progress in reducing and other drugs. opium production is under threat, owing to an upswing in opium poppy cultivation during the 2009 growing 507. -

01012100 Pure-Bred Horses 0 0 0 0 0 01012900 Lives Horses, Except
AR BR UY Mercosu PY applied NCM Description applied applied applied r Final Comments tariff tariff tariff tariff Offer 01012100 Pure-bred horses 0 0 0 0 0 01012900 Lives horses, except pure-bred breeding 2 2 2 2 0 01013000 Asses, pure-bred breeding 4 4 4 4 4 01019000 Asses, except pure-bred breeding 4 4 4 4 4 01022110 Purebred breeding cattle, pregnant or lactating 0 0 0 0 0 01022190 Other pure-bred cattle, for breeding 0 0 0 0 0 Other bovine animals for breeding,pregnant or 01022911 lactating 2 2 2 2 0 01022919 Other bovine animals for breeding 2 2 2 2 4 01022990 Other live catlle 2 2 2 2 0 01023110 Pure-bred breeding buffalo, pregnant or lactating 0 0 0 0 0 01023190 Other pure-bred breeding buffalo 0 0 0 0 0 Other buffalo for breeding, ex. pure-bred or 01023911 pregnant 2 2 2 2 0 Other buffalo for breeding, except pure-bred 01023919 breeding 2 2 2 2 4 01023990 Other buffalos 2 2 2 2 0 01029000 Other live animals of bovine species 0 0 0 0 0 01031000 Pure-bred breedig swines 0 0 0 0 0 01039100 Other live swine, weighing less than 50 kg 2 2 2 2 0 01039200 Other live swine, weighing 50 kg or more 2 2 2 2 0 01041011 Pure-bred breeding, pregnant or lactating, sheep 0 0 0 0 0 01041019 Other pure-bred breeding sheep 0 0 0 0 0 01041090 Others live sheep 2 2 2 2 0 01042010 Pure-bred breeding goats 0 0 0 0 0 01042090 Other live goats 2 2 2 2 0 Fowls spec.gallus domestic.w<=185g pure-bred 01051110 breeding 0 0 0 0 0 Oth.live fowls spec.gall.domest.weig.not more than 01051190 185g 2 2 2 2 0 01051200 Live turkeys, weighing not more than 185g 2 2 -

SENATE BILL No. 52
As Amended by Senate Committee Session of 2017 SENATE BILL No. 52 By Committee on Public Health and Welfare 1-20 1 AN ACT concerning the uniform controlled substances act; relating to 2 substances included in schedules I, II and V; amending K.S.A. 2016 3 Supp. 65-4105, 65-4107 and 65-4113 and repealing the existing 4 sections. 5 6 Be it enacted by the Legislature of the State of Kansas: 7 Section 1. K.S.A. 2016 Supp. 65-4105 is hereby amended to read as 8 follows: 65-4105. (a) The controlled substances listed in this section are 9 included in schedule I and the number set forth opposite each drug or 10 substance is the DEA controlled substances code which has been assigned 11 to it. 12 (b) Any of the following opiates, including their isomers, esters, 13 ethers, salts, and salts of isomers, esters and ethers, unless specifically 14 excepted, whenever the existence of these isomers, esters, ethers and salts 15 is possible within the specific chemical designation: 16 (1) Acetyl fentanyl (N-(1-phenethylpiperidin-4-yl)- 17 N-phenylacetamide)......................................................................9821 18 (2) Acetyl-alpha-methylfentanyl (N-[1-(1-methyl-2-phenethyl)-4- 19 piperidinyl]-N-phenylacetamide)..................................................9815 20 (3) Acetylmethadol.............................................................................9601 21 (4) AH-7921 (3.4-dichloro-N-[(1- 22 dimethylaminocyclohexylmethyl]benzamide)...............................9551 23 (4)(5) Allylprodine...........................................................................9602 -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

Opioid Receptorsreceptors
OPIOIDOPIOID RECEPTORSRECEPTORS defined or “classical” types of opioid receptor µ,dk and . Alistair Corbett, Sandy McKnight and Graeme Genes encoding for these receptors have been cloned.5, Henderson 6,7,8 More recently, cDNA encoding an “orphan” receptor Dr Alistair Corbett is Lecturer in the School of was identified which has a high degree of homology to Biological and Biomedical Sciences, Glasgow the “classical” opioid receptors; on structural grounds Caledonian University, Cowcaddens Road, this receptor is an opioid receptor and has been named Glasgow G4 0BA, UK. ORL (opioid receptor-like).9 As would be predicted from 1 Dr Sandy McKnight is Associate Director, Parke- their known abilities to couple through pertussis toxin- Davis Neuroscience Research Centre, sensitive G-proteins, all of the cloned opioid receptors Cambridge University Forvie Site, Robinson possess the same general structure of an extracellular Way, Cambridge CB2 2QB, UK. N-terminal region, seven transmembrane domains and Professor Graeme Henderson is Professor of intracellular C-terminal tail structure. There is Pharmacology and Head of Department, pharmacological evidence for subtypes of each Department of Pharmacology, School of Medical receptor and other types of novel, less well- Sciences, University of Bristol, University Walk, characterised opioid receptors,eliz , , , , have also been Bristol BS8 1TD, UK. postulated. Thes -receptor, however, is no longer regarded as an opioid receptor. Introduction Receptor Subtypes Preparations of the opium poppy papaver somniferum m-Receptor subtypes have been used for many hundreds of years to relieve The MOR-1 gene, encoding for one form of them - pain. In 1803, Sertürner isolated a crystalline sample of receptor, shows approximately 50-70% homology to the main constituent alkaloid, morphine, which was later shown to be almost entirely responsible for the the genes encoding for thedk -(DOR-1), -(KOR-1) and orphan (ORL ) receptors. -

Problems of Drug Dependence 1980 Proceedings of the 42Nd Annual Scientific Meeting the Committee on Problems of Drug Dependence
National Institute on Drug Abuse MONOGRAPH SERIES Problems of Drug Dependence 1980 Proceedings of the 42nd Annual Scientific Meeting The Committee on Problems of Drug Dependence, Inc. U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES • Public Health Service • Alcohol, Drug Abuse, and Mental Health Administration Problems of Drug Dependence, 1980 Proceedings of the 42nd Annual Scientific Meeting, The Committee on Problems of Drug Dependence, Inc. Editor: Louis S. Harris, Ph.D. NIDA Research Monograph 34 February 1981 DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Alcohol, Drug Abuse, and Mental Health Administration National Institute on Drug Abuse Division of Research 5600 Fishers Lane Rockville, Maryland 20857 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 The NIDA Research Monograph series is prepared by the Division of Research of the National Institute on Drug Abuse. Its primary objective is to provide critical reviews of research problem areas and techniques, the content of state-of-the-art conferences, integrative research reviews and significant original research. Its dual publication emphasis is rapid and targeted dissemination to the scientific and professional community. Editorial Advisory Board Avram Goldstein, M.D. Addiction Research Foundation Palo Alto, California Jerome Jaffe, M.D. College of Physicians and Surgeons Columbia University, New York Reese T. Jones, M.D. Langley Porter Neuropsychiatric Institute University of California San Francisco, California William McGlothlin, Ph.D. Deportment of Psychology, UCLA Los Angeles, California Jack Mendelson, M.D. Alcohol and Drug Abuse Research Center Harvard Medical School McLean Hospital Belmont, Massachusetts Helen Nowlis, Ph.D. Office of Drug Education, DHHS Washington, D.C Lee Robins, Ph.D. -

Research Brief New Reports, Bills and Updates of Latest Research
Research Brief New reports, bills and updates of latest research Parliamentary Library and Information Service Department of Parliamentary Services ISSN 1836-7828 (Print) 1836-8050 (Online) Number 3 February 2014 Drugs, Poisons and Controlled Substances (Poppy Cultivation and Processing) Amendment Bill 2013 This Research Brief includes the following sections: Introduction .............................................................................................................. 1 1. Second Reading Speech ...................................................................................... 1 2. Background ........................................................................................................... 2 History of the Opium Poppy and the Poppy Industry ........................................................... 2 Development of the Tasmanian Poppy Industry ..................................................................... 3 Regulation of the Poppy Industry and the Thebaine Poppy .................................................. 5 The Tasmanian Poppy Industry Today and Processor Plans for Expansion ...................... 7 Expanding the Poppy Industry to Victoria ................................................................................ 8 3. The Bill ................................................................................................................... 9 4. Other Jurisdiction ............................................................................................. 11 Appendix 1. Description of Morphine, -

Drugs of Abuseon September Archived 13-10048 No
U.S. DEPARTMENT OF JUSTICE DRUG ENFORCEMENT ADMINISTRATION WWW.DEA.GOV 9, 2014 on September archived 13-10048 No. v. Stewart, in U.S. cited Drugs of2011 Abuse EDITION A DEA RESOURCE GUIDE V. Narcotics WHAT ARE NARCOTICS? Also known as “opioids,” the term "narcotic" comes from the Greek word for “stupor” and originally referred to a variety of substances that dulled the senses and relieved pain. Though some people still refer to all drugs as “narcot- ics,” today “narcotic” refers to opium, opium derivatives, and their semi-synthetic substitutes. A more current term for these drugs, with less uncertainty regarding its meaning, is “opioid.” Examples include the illicit drug heroin and pharmaceutical drugs like OxyContin®, Vicodin®, codeine, morphine, methadone and fentanyl. WHAT IS THEIR ORIGIN? The poppy papaver somniferum is the source for all natural opioids, whereas synthetic opioids are made entirely in a lab and include meperidine, fentanyl, and methadone. Semi-synthetic opioids are synthesized from naturally occurring opium products, such as morphine and codeine, and include heroin, oxycodone, hydrocodone, and hydromorphone. Teens can obtain narcotics from friends, family members, medicine cabinets, pharmacies, nursing 2014 homes, hospitals, hospices, doctors, and the Internet. 9, on September archived 13-10048 No. v. Stewart, in U.S. cited What are common street names? Street names for various narcotics/opioids include: ➔ Hillbilly Heroin, Lean or Purple Drank, OC, Ox, Oxy, Oxycotton, Sippin Syrup What are their forms? Narcotics/opioids come in various forms including: ➔ T ablets, capsules, skin patches, powder, chunks in varying colors (from white to shades of brown and black), liquid form for oral use and injection, syrups, suppositories, lollipops How are they abused? ➔ Narcotics/opioids can be swallowed, smoked, sniffed, or injected. -
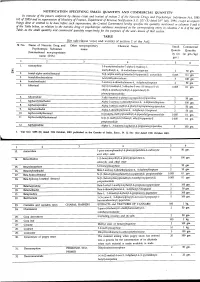
NOTIFICATION SPECIFYING SMALL QUANTITY and COMMERCIAL QUANTITYI Ly of the Pozoersconferred (Xxiiiil
NOTIFICATION SPECIFYING SMALL QUANTITY AND COMMERCIAL QUANTITYI ly of the pozoersconferred (xxiiiil . - lleyise by clansesfuiia) and of section2 of the Nnrcotic Drrtgsnnd pnlchotropic SrtbstancesAct, 19g5 (61 of 1985)and in supersessionof Ministry of Ftnnnce, (D Departmentof'Reuenue Noiification s.o.527 aotra i'6tt,1uli, tssi, exceptas respects things doneor omitted to be done .beforesuch supersession,the Cential Gooernmenihereby spectfiesthe qtnntitrl ,nlnt'ionedin coltnnnsS and 6 of the Tablebelow, in relationto.the narcoticdrug or psychotropicsubstnnce mentioned.ii tlr', ,1urrponding entnl in columns2 to 4 of thesaid Table,as the small quantitrl nnd commercialquantity respectiailyfor the purposesof the said clnuse:sof ttrit seciion. TABLE [Seesub-clause vii(a) and xxiii(a) of section 2 of the Act] Sl No. Name of Narcotic Drug and Other non-proprietary Chemical Name Small Commercial Psychotropic Substance name Quanti- Quantity (Intemational non-proprietary ity (in (in gm./kg.) name (INN) Acetorphine 3-0-acetyltetrahydro-7-alpha-(l-hydroxy-l- methylbutyt)-o, l4-endoetheno-onpavine $ 50 9.. 1\) Acetyl-alpha-methylfen N-[-(alpha-methylphenethyl)-4-piperidy]l acetanilide 0.1 g-. J. Acetyldihydrocodeine 100 4. Acetylmethadol 3-acetoxy-6-dimethylamino-4, 4 lheptane 50 gm. 5. Alfentanil -ethyl-4, N-[1-[2-( 5-dihydro-S-oxo-lH-tetrazol-t-yt) 01 gm. ethyll-4-(methoxymethyl)-4-piperidinyll -N- ylpropanamide Allyprodine 3-allyl Jmethyl-4-phenyl-4 Alpha-3-acetoxy-6-dimethylamino-4,Ld lheptane 100 gm Alpha-3-ethyl-l-methyl-4-phenyl-4-propionox 50 gm. 9.7' AlphamethadolArPnametnaool Alpha-6-dimethylamino-4, 4-diphenyl-3-heptanol 2 S0 gm. 10. Alphu-*"thylf"ntur,yl 11. -

Tennessee Drug Statutes Chart
Tennessee Drug Statutes Chart Tennessee Code: Title 39 Criminal Code SCHEDULE I OFFENSES/PENALTIES ENHANCEMENTS/ (*All sentences are for BENEFIT RESTRICTIONS standard offenders. Enhancement/mitigating factors may increase/reduce sentence. See sentencing statutes in appendix) 39-17-405 Criteria 39-17-417(b) (1) High potential for abuse; (2) Manufacture, delivery, No accepted med. Use in US or sale, possession w/ lacks accepted safety for med use intent (p.w.i.) of Schedule I Class B felony: 8-12yrs; <$100,000 39-17-406 Substances 3-17-417(i) Manufacture, (b) Opiates delivery, p.w.i. of heroin (c) Opium derivatives: E.g., (>15g); morphine heroin, codeine compounds, (>15g); hydromorphone morphine compounds, etc. (>5g); LSD (>5g); (d) Hallucinogenic substances: cocaine (>26g); E.g., MDMA, mescaline, DMT, pentazocine & peyote, LSD, psilocybin, synthetic tripelennamine (>5g); THC, etc. PCP (>30g); (e) Depressants: e.g., GHB, barbiturates (>100g); Qualuudes phenmetrazine (>50g); (f) Stimulants: E.g., fenethylline, amphetamine/ BZP methamphetamine (>26g); peyote (>1000g); Other Schedule I or II substances (>200g) Class B felony: 8-12yrs; <$200,000 SCHEDULE II 1 Tennessee Drug Statutes Chart Tennessee Code: Title 39 Criminal Code 39-17-407 Criteria 3-17-417(j) Manufacture, (1) high potential for abuse; (2) delivery, p.w.i. of heroin accepted med use in US w/ severe (>150g); morphine restrictions; and (3) abuse may (>150g); lead to severe psych or phys hydromorphone (>50g); dependence LSD (>50g); cocaine (>300g); pentazocine & tripelennamine (>50g); PCP (>300g); barbiturates (>1000g); phenmetrazine (>500g); amphetamine/ methamphetamine (>300g); peyote (>10000g); Other Schedule I or II substances (>2000g) Class A felony; 15-25yrs; <$500,000 39-17-408 Substances 39-17-417(c)(1) (b) Narcotics derived from Manufacture, delivery, vegetable origin or chemical p.w.i.