Patterns of Composition, Richness and Phylogenetic Diversity of Woody
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

View/Download
Research Article ISSN 2641-4295 Food Science & Nutrition Research Leguminous Flour from the Native Argentinian Forest, Their Contribution to Antioxidant Defense Generoso S*, Costa K, Rosas D, Lescano N and Macias S *Correspondence: Generoso S, Institute of Food Science and Technology (ICyTA), Institute of Food Science and Technology (ICyTA), Faculty of Faculty of Agronomy and Agroindustries, National University of Agronomy and Agroindustries, National University of Santiago del Santiago del Estero, Santiago del Estero, Argentina. Estero, Santiago del Estero, Argentina. Received: 20 January 2018; Accepted: 12 February 2019 Citation: Generoso S, Costa K, Rosas D, et al. Leguminous Flour from the Native Argentinian Forest, Their Contribution to Antioxidant Defense. Food Sci Nutr Res. 2019; 2(1): 1-5. ABSTRACT The oxygen-reactive species may generate oxidative stress, which could result in degenerative diseases. Antioxidant mechanisms usually act in a coordinated way, and they are grouped in two defense systems: enzymatic and non- enzymatic system. The metallo-dependent enzymes of the organism and the natural substances present in legumes have the capacity to delay, decrease or inhibit the oxidative processes. This work aimed to evaluate the bioaccessibility of zinc and bioactive compounds of flour obtained from Argentinian native forest fruits corresponding to the family of leguminous plants: white carob (Prosopis alba) and Chañar (Geoffroea decorticans), in order to use them in human food. The study was done on carob flour (CF) and chañar flour (CHF). The minerals were quantified by atomic absorption spectrometry. Bioaccessibility (D%) was estimated by dialysate percentage after in vitro digestion. The potential contribution (PC) was calculated. Their phenolic concentrations were obtained using the Folin Ciocalteu method and their antioxidant activity was evaluated in vitro using the radical DPPH (1,1-diphenil-2-pichrihydracil) and expressed as the percentage of the trapping capacity against DPPH. -

Nutraceutical Properties and Safety Evaluation of Fruits and Arrope Of
ition & F tr oo u d N f S o c l i e a n n c r e u s o J Journal of Nutrition & Food Sciences Reynoso et al., J Nutr Food Sci 2016, 6:2 ISSN: 2155-9600 DOI: 10.4172/2155-9600.1000485 Research Article Open Access Nutraceutical Properties and Safety Evaluation of Fruits and Arrope of Geoffroea decorticans (Chañar) Reynoso MA1*, Sánchez Riera A1 and Vera NR2 1Cátedra de Farmacodinamia. Facultad de Bioquímica, Química y Farmacia, Universidad Nacional de Tucumán, Ayacucho 471, 4000, Tucumán, Argentina 2Cátedera de Farmacoquímica. Facultad de Bioquímica, Química y Farmacia, Universidad Nacional de Tucumán, Ayacucho 471, 4000, Tucumán, Argentina *Corresponding author: Reynoso MA, Cátedra de Farmacodinamia. Facultad de Bioquímica, Química y Farmacia, Universidad Nacional de Tucumán, Ayacucho 471, 4000, Tucumán, Argentina, Tel: 54 381 424-7752; Fax: 54 0381 4293071; E-mail: [email protected] Received date: Mar 01, 2016; Accepted date: Mar 25, 2016; Published date: Mar 31, 2016 Copyright: © 2016 Reynoso MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Context: Geoffroea decorticans (chañar) fruits and its derivate product (arrope) have been traditionally used as food and in folk medicine for the treatment of a wide variety of diseases including bronchopulmonary disorder. Objective: The objective of this study is to evaluate the antitussive, expectorant and anti-inflammatory effects and safety of aqueous extract (AE) and arrope (Ar) of chañar. -

GRAS Notice (GRN) No. 903, Quillaia Extract Type 2
GRAS Notice (GRN) No. 903 https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory NATUREX• part of Givaudan 'fR1@CG~nill~(jJ) 07 January 2020 JAN 1 5 2020 Dr. Paulette Gaynor FOOD OF, ,Li,; UF L ADDITIVE SAFETY Office of Food Additive Safety (HFS-200) __J Center for Food Safety and Applied Nutrition (CFSAN) Food and Drug Administration 5001 Campus Drive College Park, MD 20740 USA Dear Dr. Gaynor: Re: GRAS Notice for Qulllaia Extract Type 2 In accordance with 21 CFR §170 Subpart E consisting of§§ 170.203 through 170.285, Naturex SA (250 rue Pierre Bayle, BP 81218 - 84911, Avignon, Cedex 9, France], as the notifier, is submitting one hard copy and one electronic copy (on CD) of the GRAS Notice for quillaia extract type 2, containing all data and lnfonnation supporting the company's conclusion that quillaia extract type 2 is GRAS on the basis of scientific procedures, for use in specified conventional food and beverage products across multiple categories; these food uses of quillaia extract type 2 are therefore not subject to the premarket approval requirements of the Federal Food, Drug and Cosmetic Act. Information setting forth the basis for Naturex's GRAS conclusion, as well as a consensus opinion of an independent panel of experts, also are enclosed for review by the agency. I certify that the enclosed electronic files were scanned for viruses prior to submission and are thus certified as being virus-free using Symantec Endpoint Protection 12.1.4. Should you have any questions or concerns regarding this GRAS Notice, please do not hesitate to contact me at any point during the review process so that we may provide a response in a timely manner. -
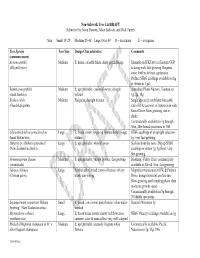
Recommended Non-Sidewalk Tree List DRAFT
Non-Sidewalk Tree List DRAFT Submitted by Jason Dewees, Mike Sullivan, and Dick Turner Size: Small 15-25’ Medium 25-40’ Large Over 40’ D = deciduous E = evergreen Tree Species Tree Size Design Characteristics Comments (common name) Acmena smithii Medium E; dense, colorful fruits; shiny green foliage Example on JFK Drive in Eastern GGP (lilly-pilly tree) is doing well; fast-growing. Requires some fertility for best appearance. Profuse SFBG seedlings available to dig or obtain in 1 gal. Banksia integrifolia Medium E; upright habit; creamy flowers; drought Australian Plants Nursery, Ventura, in (coast banksia) tolerant 1g, 5g, 15g Brahea edulis Medium Fan palm; drought tolerant Single specimen established on north (Guadalupe palm) side of JFK just west of intersection with Kezar Drive. Slow growing, sun or shade. Commercially available in 1g through 36in, 48in boxed specimens to 15ft Chiranthodendron pentadactylon Large E; broad crown; large red flowers, bold foliage SFBG seedlings of an upright selection: (hand flower tree) texture 1g; very fast-growing Hoheria sp. (Hoheria populnea? Large E; upright habit; white flowers Suckers from the roots. Dig up SFBG (New Zealand lacebark)) seedlings or obtain 1g, 5g there; very fast-growing Hymenosporum flavum Medium E; upright habit; yellow flowers; fast growing Boething, Valley Crest; commercially (sweetshade) available in 24in & 36in; fast-growing. Jubaea chilensis Large Feather palm; broad crown of leaves; silvery Magnificent specimen at JFK & Fuchsia (Chilean palm) trunk; sun-loving Drive; drought-tolerant, prefers sun. Slow-growing until trunking phase, then moderate growth speed. Commercially available in 5g through 20ft B&B specimens Leptospermum scoparium ‘Helene Small E; broad, low crown; pink flowers; often multi- Suncrest Nurseries 5g Strybing’ (New Zealand tea tree) trunked Metrosideros robusta Large E; broad dense crown; showy red flowers in SFBG Nursery seedlings available in 1g (northern rata) summer; slow & unusual but very well-adapted Michelia/Magnolia champaca or M. -

Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1
Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1 Authors: Jiang, Wei, He, Hua-Jie, Lu, Lu, Burgess, Kevin S., Wang, Hong, et. al. Source: Annals of the Missouri Botanical Garden, 104(2) : 171-229 Published By: Missouri Botanical Garden Press URL: https://doi.org/10.3417/2019337 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Downloaded From: https://bioone.org/journals/Annals-of-the-Missouri-Botanical-Garden on 01 Apr 2020 Terms of Use: https://bioone.org/terms-of-use Access provided by Kunming Institute of Botany, CAS Volume 104 Annals Number 2 of the R 2019 Missouri Botanical Garden EVOLUTION OF ANGIOSPERM Wei Jiang,2,3,7 Hua-Jie He,4,7 Lu Lu,2,5 POLLEN. 7. NITROGEN-FIXING Kevin S. Burgess,6 Hong Wang,2* and 2,4 CLADE1 De-Zhu Li * ABSTRACT Nitrogen-fixing symbiosis in root nodules is known in only 10 families, which are distributed among a clade of four orders and delimited as the nitrogen-fixing clade. -

(Gillies Ex Hook. & Arn.) Burkart Var. Decorticans
Foresta Veracruzana ISSN: 1405-7247 [email protected] Recursos Genéticos Forestales México Pece, Marta G.; Sobrero, María T.; Acosta, Marcia; Rossi, Fernando TRATAMIENTOS PREGERMINATIVOS EN Geoffroea decorticans (Gillies ex Hook. & Arn.) Burkart var. decorticans Foresta Veracruzana, vol. 16, núm. 2, septiembre-, 2014, pp. 31-36 Recursos Genéticos Forestales Xalapa, México Disponible en: http://www.redalyc.org/articulo.oa?id=49732560004 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Foresta Veracruzana 16(2):31-36. 2014. 31 TRATAMIENTOS PREGERMINATIVOS EN Geoffroea decorticans (Gillies ex Hook. & Arn.) Burkart var. decorticans Pregerminative treatments in Geoffroea decorticans (Gillies ex Hook. & Arn.) Burkart var. decorticans Marta G. Pece1, María T. Sobrero2, Marcia Acosta1 y Fernando Rossi1 Resumen El objetivo de este trabajo fue evaluar el efecto de la escarificación sobre la germinación de los frutos de G. decorticans (chañar), especie nativa del parque chaqueño. Los mismos fueron recolectados de árboles ubicados en el departamento Pellegrini (Santiago del Estero, Argentina). Previa separación del mesocarpio del resto del fruto, después de 24 hs de maceración en agua, se aplicaron los siguientes tratamientos de escarificación: frutos (testigo); semillas (T1); frutos despuntados y con incisiones longitudinales (T2); fruto despuntados y sumergidos en agua durante 4 hs (T3) y 6 hs (T4). El ensayo se condujo en germinadores que fueron colocados en cámaras de crecimiento a 30 ºC con un fotoperiodo de 14 hs de luz. -

Geoffroea Decorticans (Gill., Ex Hook. & Arn.) Burkart
FICHA DE ANTECEDENTES DE ESPECIE Nombre Científico (nombre de la especie en latín) Geoffroea decorticans (Gill., ex Hook. & Arn.) Burkart Nombre común (nombre de uso habitual que se le asigna a la especie, puede ser más de uno) Chañar Taxonomía (nombre en latín de las categorías taxonómicas a las que pertenece esta especie) Reino: Plantae Orden: Fabales Phyllum/División: Magnoliophyta Familia: Fabaceae Clase: Magnoliopsida Género: Geoffroea Sinonimia (otros nombres científicos que la especie ha tenido, pero actualmente ya no se usan) Gourliea chilensis Clos, Gourliea decorticans Hook. & Arn., Gourliea spinosa Skeels, Lucuma spinosa Molina Antecedentes Generales (breve descripción de los ejemplares, incluida características físicas, reproductivas u otras características relevantes de su historia natural. Se debería incluir también aspectos taxonómicos, en especial la existencia de subespecies o variedades. Recuerde poner las citas bibliográficas) Geoffroea decorticans, se comporta como arbusto o árbol de hasta 7 m de alto (cuando está aislado) y cuando crece en bosquecillos densos generalmente crece aproximadamente 2 m de altura (Martínez 1989). Presenta tronco tortuoso ramificado de 20-40 cm de diámetro (y de 10 a 15 cm de diámetro cuando forma bosquecillos, Iglesias y Barchuk, 2010), revestido de fajas longitudinales de ritidoma en vías de desprendimiento en los individuos adultos, ramas y ramitas grises que en la mayoría de los casos terminan en una espina dura y punzante. Las hojas son caducas, compuestas, imparipinadas, alternas o fasciculadas sobre braquiblastos pequeños axilares, raquis incluyendo pecíolo de 1,5-6 cm de largo, foliólos 5-11 por hoja, opuestos o subpuesto, subsésiles, con o sin par terminal, oblongo u oblongo-elípticos obtusos y emarginados; pubérulos cuando joven y glabros cuando adultos; pinatinervios, algo duros, miden 5-15 mm de largo por 3-8 mm de ancho. -

Quillaja Saponaria (Molina) Extracts Inhibits in Vitro Piscirickettsia Salmonis Infections
animals Article Quillaja saponaria (Molina) Extracts Inhibits In Vitro Piscirickettsia salmonis Infections Hernán Cañon-Jones 1,* , Hernán Cortes 2, Mario Castillo-Ruiz 3,4 , Trinidad Schlotterbeck 5 and Ricardo San Martín 5 1 Núcleo de Investigación Aplicada en Ciencias Veterinarias y Agronómicas, Facultad de Medicina Veterinaria y Agronomía, Universidad de Las Américas, Santiago 7500975, Chile 2 Desert King Chile, Viña del Mar 2420505, Chile; [email protected] 3 Escuela de Química y Farmacia, Facultad de Medicina, Universidad Andres Bello, Santiago 8370146, Chile; [email protected] 4 Departamento de Ciencias Químicas y Biológicas, Facultad de Ciencias de la Salud, Universidad Bernardo O Higgins, Santiago 8370993, Chile 5 Saponin Research Center, Santiago 7510132, Chile; [email protected] (T.S.); [email protected] (R.S.M.) * Correspondence: [email protected] Received: 15 September 2020; Accepted: 10 November 2020; Published: 3 December 2020 Simple Summary: Bacterial diseases causes massive mortalities in aquaculture and antibiotic use remains the main measure to keep these under control. Pisciricketssia salmonis, an intracellular bacterium only present in Chile, produces high mortalities in farmed salmon and is currently the main reason for using antimicrobials compared to other salmon-producing countries such as Norway. Environmental and antimicrobial resistance concerns have been raised by the local and global public and society, although no scientific evidence has demonstrated such an impact. Thus, there is a constant search for new alternatives that can complement or reduce the use of antimicrobial in intensive salmon farming. Phytochemicals such as saponins from Quillaja saponaria extracts have been proven to prevent and control diseases in other animal production systems. -

Natural Surfactant Saponin from Tissue of Litsea Glutinosa and Its Alternative Sustainable Production
plants Article Natural Surfactant Saponin from Tissue of Litsea glutinosa and Its Alternative Sustainable Production Jiratchaya Wisetkomolmat 1,2, Ratchuporn Suksathan 3, Ratchadawan Puangpradab 3, Keawalin Kunasakdakul 4, Kittisak Jantanasakulwong 5,6, Pornchai Rachtanapun 5,6 and Sarana Rose Sommano 2,5,7,* 1 Interdisciplinary Program in Biotechnology, Graduate School, Chiang Mai University, Chiang Mai 50200, Thailand; [email protected] 2 Plant Bioactive Compound Laboratory, Department of Plant and Soil Sciences, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand 3 Research and Product Development, Department of Research and Conservation, Queen Sirikit Botanic Garden, The Botanical Garden Organisation, Chiang Mai 50180, Thailand; [email protected] (R.S.); [email protected] (R.P.) 4 Department of Entomology and Plant Pathology, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand; [email protected] 5 Cluster of Agro Bio-Circular-Green Industry (Agro BCG), Chiang Mai University, Chiang Mai 50100, Thailand; [email protected] (K.J.); [email protected] (P.R.) 6 Division of Packaging Technology, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand 7 Innovative Agriculture Research Centre, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand * Correspondence: [email protected]; Tel.: +66-53944040 Received: 8 October 2020; Accepted: 6 November 2020; Published: 9 November 2020 Abstract: In this research, we assessed the detergency properties along with chemical characteristic of the surfactant extracts from the most frequently cited detergent plants in Northern Thailand, namely, Sapindus rarak, Acacia concinna, and Litsea glutinosa. Moreover, as to provide the sustainable option for production of such valuable ingredients, plant tissue culture (PTC) as alternative method for industrial metabolite cultivation was also proposed herein. -

Contributions to the Solution of Phylogenetic Problem in Fabales
Research Article Bartın University International Journal of Natural and Applied Sciences Araştırma Makalesi JONAS, 2(2): 195-206 e-ISSN: 2667-5048 31 Aralık/December, 2019 CONTRIBUTIONS TO THE SOLUTION OF PHYLOGENETIC PROBLEM IN FABALES Deniz Aygören Uluer1*, Rahma Alshamrani 2 1 Ahi Evran University, Cicekdagi Vocational College, Department of Plant and Animal Production, 40700 Cicekdagi, KIRŞEHIR 2 King Abdulaziz University, Department of Biological Sciences, 21589, JEDDAH Abstract Fabales is a cosmopolitan angiosperm order which consists of four families, Leguminosae (Fabaceae), Polygalaceae, Surianaceae and Quillajaceae. The monophyly of the order is supported strongly by several studies, although interfamilial relationships are still poorly resolved and vary between studies; a situation common in higher level phylogenetic studies of ancient, rapid radiations. In this study, we carried out simulation analyses with previously published matK and rbcL regions. The results of our simulation analyses have shown that Fabales phylogeny can be solved and the 5,000 bp fast-evolving data type may be sufficient to resolve the Fabales phylogeny question. In our simulation analyses, while support increased as the sequence length did (up until a certain point), resolution showed mixed results. Interestingly, the accuracy of the phylogenetic trees did not improve with the increase in sequence length. Therefore, this study sounds a note of caution, with respect to interpreting the results of the “more data” approach, because the results have shown that large datasets can easily support an arbitrary root of Fabales. Keywords: Data type, Fabales, phylogeny, sequence length, simulation. 1. Introduction Fabales Bromhead is a cosmopolitan angiosperm order which consists of four families, Leguminosae (Fabaceae) Juss., Polygalaceae Hoffmanns. -

Leaf Anatomy of Beilschmiedia (Lauraceae) in the Neotropics
Article Leaf anatomy of Beilschmiedia (Lauraceae) in the neotropics Sachiko Nishida1 and David C. Christophel2 1 Division of Phylogenetics, Museum of Nature and Human Activities, Hyogo, Yayoigaoka 6, Sanda, 669-1546, Japan 2 Botany Department, University of Adelaide, Adelaide, S.A. 5005, Australia Abstract Beilschmiedia comprises 28 species in the neotropics, but the relationships among the species were not well known because of the similarity in their reproductive morphology. This study employed leaf anatomy including studies of venation patterns, cuticle and leaf sections to discuss the relationships. As a result, five groups are recognized by cuticular characters, and the groups are compatible with the other leaf characters, phyllotaxis and geographic distributions. The five groups are mainly characterized by; 1) alternate leaves, fine venation pattern, epidermal cells with smooth or beaded cell walls, and vascular bundles in the midrib arranged in a ring, 2) opposite leaves, coarse venation pattern, epidermal cells with dotted or granular periclinal walls and mostly branched anticlinal walls, and vascular bundles in the midrib arranged in a ring, 3) opposite leaves, coarse venation pattern, butterfly-shaped stomatal ledges, and vascular bundles in the midrib arranged in a flattened arc, 4) opposite leaves, coarse venation pattern, epidermal cells larger on the adaxial surface than the abaxial surface, and vascular bundles in the midrib arranged in a flattened arc, 5) opposite leaves, coarse venation pattern, box- shaped stomatal ledges, and vascular bundles in the midrib arranged in a flattened arc. Key words: Beilschmiedia, leaf anatomy, venation, cuticle, vascular bundle Introduction botanists. Christophel and Rowett (1996) recently published a leaf and cuticle atlas of Australian Lauraceae, Beilschmiedia is one of the largest genera of Lauraceae, which includes the leaf architecture and cuticular features comprising about 250 tree or shrub species. -

Combined Phylogenetic Analyses Reveal Interfamilial Relationships and Patterns of floral Evolution in the Eudicot Order Fabales
Cladistics Cladistics 1 (2012) 1–29 10.1111/j.1096-0031.2012.00392.x Combined phylogenetic analyses reveal interfamilial relationships and patterns of floral evolution in the eudicot order Fabales M. Ange´ lica Belloa,b,c,*, Paula J. Rudallb and Julie A. Hawkinsa aSchool of Biological Sciences, Lyle Tower, the University of Reading, Reading, Berkshire RG6 6BX, UK; bJodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3DS, UK; cReal Jardı´n Bota´nico-CSIC, Plaza de Murillo 2, CP 28014 Madrid, Spain Accepted 5 January 2012 Abstract Relationships between the four families placed in the angiosperm order Fabales (Leguminosae, Polygalaceae, Quillajaceae, Surianaceae) were hitherto poorly resolved. We combine published molecular data for the chloroplast regions matK and rbcL with 66 morphological characters surveyed for 73 ingroup and two outgroup species, and use Parsimony and Bayesian approaches to explore matrices with different missing data. All combined analyses using Parsimony recovered the topology Polygalaceae (Leguminosae (Quillajaceae + Surianaceae)). Bayesian analyses with matched morphological and molecular sampling recover the same topology, but analyses based on other data recover a different Bayesian topology: ((Polygalaceae + Leguminosae) (Quillajaceae + Surianaceae)). We explore the evolution of floral characters in the context of the more consistent topology: Polygalaceae (Leguminosae (Quillajaceae + Surianaceae)). This reveals synapomorphies for (Leguminosae (Quillajaceae + Suri- anaceae)) as the presence of free filaments and marginal ⁄ ventral placentation, for (Quillajaceae + Surianaceae) as pentamery and apocarpy, and for Leguminosae the presence of an abaxial median sepal and unicarpellate gynoecium. An octamerous androecium is synapomorphic for Polygalaceae. The development of papilionate flowers, and the evolutionary context in which these phenotypes appeared in Leguminosae and Polygalaceae, shows that the morphologies are convergent rather than synapomorphic within Fabales.