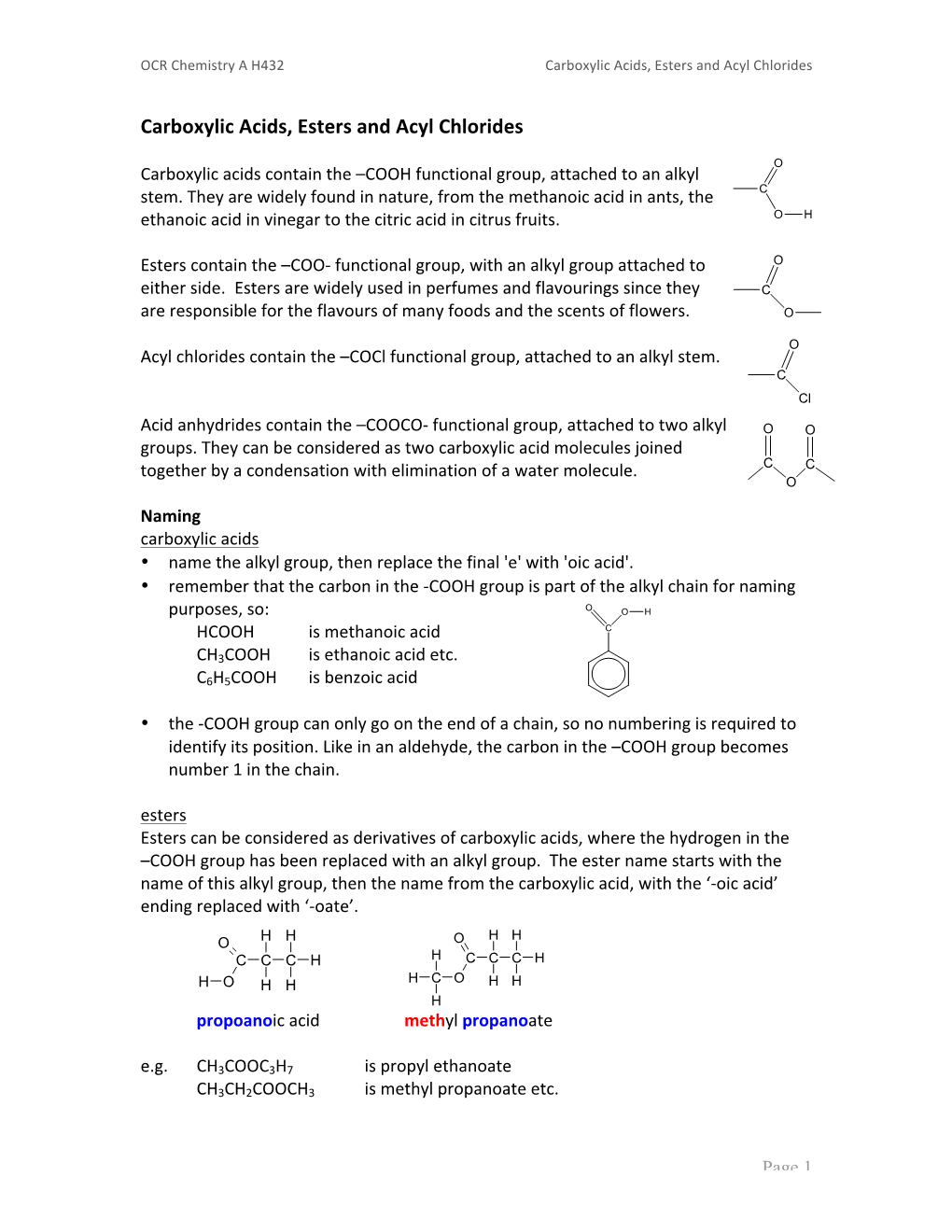
Carboxylic Acids, Esters and Acyl Chlorides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ebook for A2.2 Chemistry
eBook for A2.2 Chemistry Chemistry in Medicine 5.11. [Pages 94 – 107 of A2.2 eBook] • You will be expected to be able to explain the use of indigestion remedies to cure excess hydrochloric acid in the stomach stating the types of compounds used and writing equations for their reactions. • You will be expected to be able to use a back titration to determine the percentage of an active ingredient in an indigestion remedy and perform various calculations on the titration. • You will be expected to be able to explain how to deal with variations in the pH values of skin, explain the role of fatty acids in skin pH and explain the use of corrosive chemicals in removing warts. • You will be expected to be able to recall and explain the use of silver nitrate in the treatment of eye diseases. • You will be expected to be able to explain the action of anticancer drugs, for example cisplatin in preventing DNA replication in cancer cells and how varying the structure of cisplatin affects the efficiency of anticancer activity • You will be expected to be able to carry out titrations to determine the concentration of aspirin in solution and carry out associated calculations. • You will be expected to be able to recall the synthesis of aspirin from salicylic acid and ethanoic anhydride and compare it with the use of ethanoic acid and ethanoyl chloride and explain why the sodium salt of aspirin is often used rather than aspirin. • You will be expected to be able to explain the use of GLC linked to MS to identify drugs and to determine their purity. -

Nomenclature of Carboxylic Acid Derivatives Acid Halide Substituents
Gentilucci, Carboxylic Acid Derivatives Nomenclature of Carboxylic Acid Derivatives Gentilucci, Carboxylic Acid Derivatives Acid halides 1. Alkane + the suffix -oyl followed by the halogen. 2. Select the longest continuous carbon chain, containing the acyl group. 3. Number the carbon chain, beginning at the end nearest to the acyl group. 4. Number the substituents and write the name, listing substituents alphabetically. Acid halide substituents attached to rings are named using the suffix - carbonyl. 1 Gentilucci, Carboxylic Acid Derivatives Anhydrides 1. Symmetrical: replace the ending "acid" with "anhydride ". 2. Asymmetrical: select the longest continuous carbon chain, containing the carboxylic acid group, and derive the parent name by replacing the -e ending with -oic anhydride . 3. Number the carbon chain, beginning at the end nearest to the acyl group. 4. Number the substituents and write the name, listing substituents alphabetically. Gentilucci, Carboxylic Acid Derivatives Amides are named by replacing the ending -oic acid with -amide . 1. Select the longest continuous carbon chain, containing the acyl group, and derive the parent name by replacing the -e ending with -amide . 2. Number the carbon chain, beginning at the end nearest to the acyl group. 3. Number the substituents and write the name, listing substituents alphabetically. 4. If the nitrogen atom is further substituted, the substituents are preceded by N- to indicate that they are attached to the nitrogen. Acid halide substituents attached to rings are named using the suffix - carboxamide. 2 Gentilucci, Carboxylic Acid Derivatives Carboxylate esters 1. Select the longest continuous carbon chain containing the acyl group, and derive the parent name by replacing the -e ending with –oate . -

Carbonyl Compounds
Carbonyl Compounds What are Carbonyl Compounds? Carbonyl compounds are compounds that contain the C=O (carbonyl) group. Preparation of Aldehydes: 1. Preparation from Acid Chloride (Rosenmund Reduction): This reaction was named after Karl Wilhelm Rosenmund, who first reported it in 1918. The reaction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. The reaction, a hydrogenolysis, is catalysed by palladium on barium sulfate, which is sometimes called the Rosenmund catalyst. 2. Preparation from Nitriles: This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R- CN) using SnCl2 and HCl and quenching the resulting iminium salt ([R- + − CH=NH2] Cl ) with water (H2O). During the synthesis, ammonium chloride is also produced. The reaction is known as Stephen Aldehyde synthesis. Dr. Sumi Ganguly Page 1 3. Preparation from Grignard Reagent: When Grignard Reagent is reacted with HCN followed by hydrolysis aldehyde is produced. Preparation of Ketones: 1. Preparation from Acid Chloride (Friedel-Crafts Acylation): Acid chlorides when reacted with benzene in presence of anhydrous AlCl3, aromatic ketone are produced. However, only aromatic ketones can be prepared by following this method. In order to prepare both aromatic and aliphatic ketones acid chlorides is reacted with lithium dialkylcuprate (Gilman Reagnt). Dr. Sumi Ganguly Page 2 The lithium dialkyl cuprate is produced by the reaction of two equivalents of the organolithium reagent with copper (I) iodide. Example: 3. Preparation from Nitriles and Grignard Reagents: When Grignard Reagent is reacted with RCN followed by hydrolysis aldehyde is produced. Dr. Sumi Ganguly Page 3 Physical Characteristic of Carbonyl Compounds: 1) The boiling point of carbonyl compounds is higher than the alkanes with similar Mr. -

Derivatives of Carboxylic Acid
Derivatives of Carboxylic Acid acid chloride carboxylate nitrile amide acid anhydride ester Nomenclature of Acid Halides IUPAC: alkanoic acid → alkanoyl halide Common: alkanic acid → alkanyl halide I: 3-aminopropanoyl chloride I: 4-nitropentanoyl chloride c: b-aminopropionyl chloride c: g-nitrovaleryl chloride I: hexanedioyl chloride c: adipoyl chloride Rings: (IUPAC only): ringcarbonyl halide I: benzenecarbonyl bromide I: 3-cylcopentenecarbonyl chloride c: benzoyl bromide Nomenclature of Acid Anhydrides Acid anhydrides are prepared by dehydrating carboxylic acids acetic anhydride ethanoic acid ethanoic anhydride I: benzenecarboxylic anhydride I: butanedioic acid I: butanedioic anhydride c: benzoic andhydride c: succinic acid c: succinic anhydride Some unsymmetrical anhydrides I: ethanoic methanoic I: benzoic methanoic anhydride anhydride I: cis-butenedioic c: benzoic formic anhydride anhydride c: acetic formic anhydride Nomenclature of Esters Esters occur when carboxylic acids react with alcohols I: phenyl methanoate I: t-butyl benzenecarboxylate I: methyl ethanoate c: phenyl formate c: methyl acetate c: t-butyl benzoate I: isobutyl I: cyclobutyl 2- I: dimethyl ethanedioate cyclobutanecarboxylate methylpropanoate c: cyclobutyl a- c: dimethyl oxalate c: none methylpropionate Cyclic Esters Reaction of -OH and -COOH on same molecule produces a cyclic ester, lactone. To name, add word lactone to the IUPAC acid name or replace the -ic acid of common name with -olactone. 4-hydroxy-2-methylpentanoic acid lactone -methyl- -valerolactone Amides Product of the reaction of a carboxylic acid and ammonia or an amine. Not basic because the lone pair on nitrogen is delocalized by resonance. Classes of Amides 1 amide has one C-N bond (two N-H). 2 amide or N-substituted amide has two C-N bonds (one N-H). -

Indium Promoted-Convenient Method for Acylation of Alc이iols with Acyl Chlorides
Communications to the Editor Bull. Korean Chem. Soc. 2003, Vol. 24, No. 2 155 Indium Promoted-Convenient Method for Acylation of Alc이iols with Acyl Chlorides Dae Hyan Cho, Joong Gon Kim,f and Doo Ok Jang* Department of Chemistry, Yonsei University, Wonju 220-710, Korea ‘Biotechnology Division, Hanwha Chemical R & D Center, Daejeon 305-345, Korea Received November 9, 2002 Key Words : Indium, Alcohol, Acylation, Acyl chloride Even though various reagents for coupling of alcohols tions for the acylation of alcohols with acyl chlorides in the with carboxylic acids and transesterification of esters have presence of indium. The results are summarized in Table 1. been developed,1 there is still a great demand for a process Reaction of 2 (1 equiv) with 1 (1 equiv) in the presence of by using acyl chlorides for the acylation of alcohols in the indium (1 equiv) in CH3CN at room temperature produced case of substrates having steric hindrance or low reactivity. the corresponding ester in only 21% yield and the starting The acylation of alcohols with acyl chlorides is commonly acyl chloride and alcohol were recovered. The optimal yield carried out in the presence of tertiary amines such as 4- of the ester was attained with 3 equiv of 1 or 2 in the presence (methylamino) pyridine or 4 -pyrrolidinopyridine.2 Many of 3 equiv of indium. The solvent effect of acylation of 2 methods for the acylation of alcohols with acyl chlorides with 1 in the presence of indium was studied. The reaction have been developed using a variety of reagents.3 Most proceeded efficiently in common organic solvents such as recently, benzoylation of alcohols with lithium perchlorate DMF, Et2。,THF or CHzCh whereas non-polar solvents hase been reported.4 However, these methods have their own such as n-hexane or benzene gave poor yields of the ester. -

Part I. the Total Synthesis Of
AN ABSTRACT OF THE THESIS OF Lester Percy Joseph Burton forthe degree of Doctor of Philosophy in Chemistry presentedon March 20, 1981. Title: Part 1 - The Total Synthesis of (±)-Cinnamodialand Related Drimane Sesquiterpenes Part 2 - Photochemical Activation ofthe Carboxyl Group Via NAcy1-2-thionothiazolidines Abstract approved: Redacted for privacy DT. James D. White Part I A total synthesis of the insect antifeedant(±)-cinnamodial ( ) and of the related drimanesesquiterpenes (±)-isodrimenin (67) and (±)-fragrolide (72)are described from the diene diester 49. Hydro- boration of 49 provided the C-6oxygenation and the trans ring junction in the form of alcohol 61. To confirm the stereoselectivity of the hydroboration, 61 was convertedto both (t)-isodrimenin (67) and (±)-fragrolide (72) in 3 steps. A diisobutylaluminum hydride reduction of 61 followed by a pyridiniumchlorochromate oxidation and treatment with lead tetraacetate yielded the dihydrodiacetoxyfuran102. The base induced elimination of acetic acid preceded theepoxidation and provided 106 which contains the desired hydroxy dialdehydefunctionality of cinnamodial in a protected form. The epoxide 106 was opened with methanol to yield the acetal 112. Reduction, hydrolysis and acetylation of 112 yielded (t)- cinnamodial in 19% overall yield. Part II - Various N- acyl- 2- thionothiazolidineswere prepared and photo- lysed in the presence of ethanol to provide the corresponding ethyl esters. The photochemical activation of the carboxyl function via these derivatives appears, for practical purposes, to be restricted tocases where a-keto hydrogen abstraction and subsequent ketene formation is favored by acyl substitution. Part 1 The Total Synthesis of (±)-Cinnamodial and Related Drimane Sesquiterpenes. Part 2 Photochemical Activation of the Carboxyl Group via N-Acy1-2-thionothiazolidines. -

Derivatives of Carboxylic Acids
Acylation A4 1 DERIVATIVES OF CARBOXYLIC ACIDS ACYL (ACID) CHLORIDES - RCOCl ACID ANHYDRIDES - (RCO)2O named from corresponding acid named from corresponding acid remove -ic add -yl chloride remove acid add anhydride CH3COCl ethanoyl chloride (CH3CO)2O ethanoic anhydride C6H5COCl benzene carbonyl chloride δ− δ+ O δ− CH C O 3 δ− δ+ O δ+ CH3 C δ− CH3 C δ− Cl O bonding in acyl chlorides bonding in acid anhydrides Chemical Properties • colourless liquids which fume in moist air • acyl chlorides are more reactive than anhydrides • attacked at the positive carbon centre by nucleophiles • nucleophiles include water, alcohols, ammonia and amines • undergo addition-elimination reactions Uses of Acylation Industrially Manufacture of Cellulose acetate - making fibres Aspirin (acetyl salicylic acid) - analgaesic Ethanoic anhydride is more useful • cheaper • less corrosive • less vulnerable to hydrolysis • less dangerous reaction Laboratory Used to make carboxylic acid, esters, amines, N-substituted amines Ethanoyl chloride is used as it • is more reactive • gives a cleaner reaction Q.1 Investigate how aspirin is made industrially and in the laboratory. Why are the reagents and chemicals different? What properties of Aspirin make it such a useful drug? 2 A4 Acylation ADDITION ELIMINATION REACTIONS - OVERVIEW Mechanism • species attacked by nucleophiles at the positive carbon end of the C=O bond • the nucleophile adds to the molecule • either Cl or RCOO¯ is eliminated • a proton is removed General example - with water ACID CHLORIDES H Cl + Cl H O O -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

Nomenclature of Carboxylic Acids • Select the Longest Carbon Chain Containing the Carboxyl Group
Chapter 5 Carboxylic Acids and Esters Carboxylic Acids • Carboxylic acids are weak organic acids which Chapter 5 contain the carboxyl group (RCO2H): Carboxylic Acids and Esters O C O H O RCOOH RCO2H Chapter Objectives: O condensed ways of • Learn to recognize the carboxylic acid, ester, and related functional groups. RCOH writing the carboxyl • Learn the IUPAC system for naming carboxylic acids and esters. group a carboxylic acid C H • Learn the important physical properties of the carboxylic acids and esters. • Learn the major chemical reaction of carboxylic acids and esters, and learn how to O predict the products of ester synthesis and hydrolysis reactions. the carboxyl group • Learn some of the important properties of condensation polymers, especially the polyesters. Mr. Kevin A. Boudreaux • The tart flavor of sour-tasting foods is often caused Angelo State University CHEM 2353 Fundamentals of Organic Chemistry by the presence of carboxylic acids. Organic and Biochemistry for Today (Seager & Slabaugh) www.angelo.edu/faculty/kboudrea 2 Nomenclature of Carboxylic Acids • Select the longest carbon chain containing the carboxyl group. The -e ending of the parent alkane name is replaced by the suffix -oic acid. • The carboxyl carbon is always numbered “1” but the number is not included in the name. • Name the substituents attached to the chain in the Nomenclature of usual way. • Aromatic carboxylic acids (i.e., with a CO2H Carboxylic Acids directly connected to a benzene ring) are named after the parent compound, benzoic acid. O C OH 3 -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

Ester Synthesis Lab (Student Handout)
Name: ________________________ Lab Partner: ____________________ Date: __________________________ Class Period: ____________________ Ester Synthesis Lab (Student Handout) Lab Report Components: The following must be included in your lab book in order to receive full credit. 1. Purpose 2. Hypothesis 3. Procedure 4. Observation/Data Table 5. Results 6. Mechanism (In class) 7. Conclusion Introduction The compounds you will be making are also naturally occurring compounds; the chemical structure of these compounds is already known from other investigations. Esters are organic molecules of the general form: where R1 and R2 are any carbon chain. Esters are unique in that they often have strong, pleasant odors. As such, they are often used in fragrances, and many artificial flavorings are in fact esters. Esters are produced by the reaction between alcohols and carboxylic acids. For example, reacting ethanol with acetic acid to give ethyl acetate is shown below. + → + In the case of ethyl acetate, R1 is a CH3 group and R2 is a CH3CH2 group. Naming esters systematically requires naming the functional groups on both sides of the bridging oxygen. In the example above, the right side of the ester as shown is a CH3CH2 1 group, or ethyl group. The left side is CH3C=O, or acetate. The name of the ester is therefore ethyl acetate. Deriving the names of the side from the carboxylic acid merely requires replacing the suffix –ic with –ate. Materials • Alcohol • Carboxylic Acid o 1 o A o 2 o B o 3 o C o 4 Observation Parameters: • Record the combination of carboxylic acid and alcohol • Observe each reactant • Observe each product Procedure 1. -

N Goalby Chemrevise.Org 1 Reaction with Water
Carboxylic acid derivatives: Acyl Chlorides and Acid Anhydrides Acyl Chlorides Acid Anhydrides Acid anhydrides have a similar reactivity to O Acyl chlorides are much O acyl chlorides and therefore bring about the CH3 C more reactive than same changes in functional groups. carboxylic acids CH3 C Cl O ethanoyl chloride The main difference is the by-products. CH3 C Acyl chlorides mostly give off HCl. Acid O anhydrides give off RCOOH ethanoic anhydride. Explaining reactivity Many of the reactions of the carboxylic acid derivatives follow the pattern below with an attack by an nucleophile. O O CH3 C + :X- CH3 C + :W- On a simplistic level, the stronger the electron attracting power of ‘W’, the W X more positive the carbon, and the more attractive the carbon is to Where –W: and :W- can be –Cl and Cl- (acyl chlorides) nucleophiles. - or -OCH3 and OCH3 (esters) The relative attractive powers of the –W: are - `or -NH2 and NH2 (amides) -Cl > -OH > -OCH3 > -NH2 Therefore in the case of hydrolysis reactions, acyl chlorides are highly reactive and will be hydrolysed by weak nucleophiles such as water. Amides and Esters contain only weak electron attracting W groups and need strong nucleophiles such as hydroxide ions in NaOH to hydrolyse. This difference in reactivity is caused by a combination of two factors 1. the electronegativity of the Cl’s, N’s and O’s causing electron density to be withdrawn from the carbon making the carbon more positive and more attractive to nucleophiles- This factor makes them more reactive. 2. delocalisation of the lone pairs on these atoms into the carbonyl system which reduces the reactivity.