Efficient and Controllably Selective Preparation of Esters Using Uronium-Based Coupling Agents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

Nomenclature of Carboxylic Acids • Select the Longest Carbon Chain Containing the Carboxyl Group
Chapter 5 Carboxylic Acids and Esters Carboxylic Acids • Carboxylic acids are weak organic acids which Chapter 5 contain the carboxyl group (RCO2H): Carboxylic Acids and Esters O C O H O RCOOH RCO2H Chapter Objectives: O condensed ways of • Learn to recognize the carboxylic acid, ester, and related functional groups. RCOH writing the carboxyl • Learn the IUPAC system for naming carboxylic acids and esters. group a carboxylic acid C H • Learn the important physical properties of the carboxylic acids and esters. • Learn the major chemical reaction of carboxylic acids and esters, and learn how to O predict the products of ester synthesis and hydrolysis reactions. the carboxyl group • Learn some of the important properties of condensation polymers, especially the polyesters. Mr. Kevin A. Boudreaux • The tart flavor of sour-tasting foods is often caused Angelo State University CHEM 2353 Fundamentals of Organic Chemistry by the presence of carboxylic acids. Organic and Biochemistry for Today (Seager & Slabaugh) www.angelo.edu/faculty/kboudrea 2 Nomenclature of Carboxylic Acids • Select the longest carbon chain containing the carboxyl group. The -e ending of the parent alkane name is replaced by the suffix -oic acid. • The carboxyl carbon is always numbered “1” but the number is not included in the name. • Name the substituents attached to the chain in the Nomenclature of usual way. • Aromatic carboxylic acids (i.e., with a CO2H Carboxylic Acids directly connected to a benzene ring) are named after the parent compound, benzoic acid. O C OH 3 -

Ester Synthesis Lab (Student Handout)
Name: ________________________ Lab Partner: ____________________ Date: __________________________ Class Period: ____________________ Ester Synthesis Lab (Student Handout) Lab Report Components: The following must be included in your lab book in order to receive full credit. 1. Purpose 2. Hypothesis 3. Procedure 4. Observation/Data Table 5. Results 6. Mechanism (In class) 7. Conclusion Introduction The compounds you will be making are also naturally occurring compounds; the chemical structure of these compounds is already known from other investigations. Esters are organic molecules of the general form: where R1 and R2 are any carbon chain. Esters are unique in that they often have strong, pleasant odors. As such, they are often used in fragrances, and many artificial flavorings are in fact esters. Esters are produced by the reaction between alcohols and carboxylic acids. For example, reacting ethanol with acetic acid to give ethyl acetate is shown below. + → + In the case of ethyl acetate, R1 is a CH3 group and R2 is a CH3CH2 group. Naming esters systematically requires naming the functional groups on both sides of the bridging oxygen. In the example above, the right side of the ester as shown is a CH3CH2 1 group, or ethyl group. The left side is CH3C=O, or acetate. The name of the ester is therefore ethyl acetate. Deriving the names of the side from the carboxylic acid merely requires replacing the suffix –ic with –ate. Materials • Alcohol • Carboxylic Acid o 1 o A o 2 o B o 3 o C o 4 Observation Parameters: • Record the combination of carboxylic acid and alcohol • Observe each reactant • Observe each product Procedure 1. -

In This Handout, All of Our Functional Groups Are Presented As Condensed Line Formulas, 2D and 3D Formulas and with Nomenclature Prefixes and Suffixes (If Present)
In this handout, all of our functional groups are presented as condensed line formulas, 2D and 3D formulas and with nomenclature prefixes and suffixes (if present). Organic names are built on a foundation of alkanes, alkenes and alkynes. Those examples are presented first and you need to know those rules. The strategies can be found in Chapter 4 of our textbook (alkanes: pages 93-98, cycloalkanes 102-104, alkenes: pages 104-110, alkynes: pages 112-113 and combinations of all of them 113-115). After introducing examples of alkanes, alkenes, alkynes and combinations of them, the functional groups are presented in order of priority. A few nomenclature examples are provided for each of the functional groups. Examples of the various functional groups are presented on pages 115-135 in the textbook. Two overview pages are on pages 136-137. Some functional groups have a suffix name when they are the highest priority functional group and a prefix name when they are not the highest priority group, and these are added to the skeletal names with identifying numbers and stereochemistry terms (E and Z for alkenes, R and S for chiral centers and cis and trans for rings). Several low priority functional groups only have a prefix name. A few additional special patterns are shown on pages 98-102. The only way to learn this topic is practice (over and over). The best practice approach is to actually write out the names (on an extra piece of paper or on a white board, and then do it again). The same functional groups are used throughout the entire course. -

4.4 Formation of Esters from Carboxylic Acids and Alcohols
4.4 Formation of Esters from Carboxylic Acids and Alcohols Carboxylic acids can react with alcohols to form esters, a reaction called esterification. This is an endergonic (endothermic) reversible reaction with a high activation energy barrier in the absence of a catalyst. The esterification reaction profile is In the forward direction it is called an esterification reaction, because it produces an ester. In the reverse direction it is called a hydrolysis reaction because it breaks up the ester and adds a water molecule in the process. Note that a water molecule is removed in the process of forming the ester from the carboxylic acid and the alcohol. The water comes from removing an OH group on the carboxylic acid and combining it with a H. One can show this using “lasso” chemistry as shown 20 + H2O (One might wonder how the ester gets formed in the first place, given that it is uphill from the carboxylic acid and alcohol molecules. In fact in biological systems the carboxylic acids are not the reactive molecule itself. The carboxylic acid is activated (energy level raised) by attaching a group which raises its energy level. It is this activated carboxylic acid which reacts with the alcohol and due to the high energy of the activated carboxylic acid, this reaction is exergonic. Once formed the ester often has an activation energy barrier that is high enough that hydrolysis will not occur unless a specific esterase enzyme is present.) Draw the products of the following reactions ___________ + HOCH3 > H+ 21 + ___ H+____> __ H+___________ + HOCH2CH3 > Para aminobenzoic acid + ethanol ____> benzocaine(product) Benzocaine is commonly used as a topical ester local anesthetic to reduce the pain of sunburn and to temporarily reduce dental pain. -

Investigations of the Reactivity of Pyridine Carboxylic Acids with Diazodiphenylmethane in Protic and Aprotic Solvents. Part I
J. Serb. Chem. Soc. 70 (4) 557–567 (2005) UDC 547.821+547.595:543.878 JSCS – 3288 Original scientific paper Investigations of the reactivity of pyridine carboxylic acids with diazodiphenylmethane in protic and aprotic solvents. Part I. Pyridine mono-carboxylic acids ALEKSANDAR D. MARINKOVI]*#, SA[A @. DRMANI]#, BRATISLAV @. JOVANOVI]# and MILICA MI[I]-VUKOVI]# Department of Organic Chemistry, Faculty of Technology and Metallurgy, University of Belgrade, Karnegijeva 4, P. O. Box 3503, 11120 Belgrade, Serbia and Montenegro (e-mail: [email protected]) (Received 14 May, revised 26 July 2004) Abstract: Rate constants for the reaction of diazodiphenylmethane with isomeric pyridine carboxylic acids were determined in chosen protic and aprotic solvents at 30 °C, using the well known UV spectrophotometric method. The values of the rate constants of the investigated acids in protic solvents were higher than those in aprotic solvents. The second order rate constants were correlated with solvent pa- rameters using the Kamlet-Taft solvatochromic equation in the form: log k =logk0 + sp*+aa + bb. The correlation of the obtained kinetic data were performed by means of multiple linear regression analysis taking appropriate solvent parameters. The signs of the equation coefficients were in agreement with the postulated reaction mechanism. The mode of the influence of the solvent on the reaction rate in all the investigated acids are discussed on the basis of the correlation results. Keywords: pyridine carboxylic acids, diazodiphenylmethane, rate constants, solvent parameters, protic and aprotic solvents. INTRODUCTION The relationship between the structure of carboxylic acids and their reactivity with diazodiphenylmethane (DDM) has been studied by many authors,1,2 with particular regard to the influence of the solvent. -

Carboxylic Acids
CARBOXYLIC ACIDS 1 Carboxylic Acids Introduction Carboxylic acids are organic compounds containing the carboxyl group (-COOH), wherein the hydroxyl group (-OH) is directly attached to the carbonyl (C=O) group. Carboxylic acids constitute one of the most frequently encountered classes of organic compounds in nature. 2 Natural Carboxylic Acids A great many carboxylic acids are encountered in nature, mostly, in fruits. Indeed carboxylic acids were among the first class of organic compounds to ever be isolated from nature. Edible carboxylic acids found in citrous fruits and fermented milk generally have sharp flavours. 3 Nomenclature of Carboxylic Acids The common names of some basic carboxylic acids are derived from Latin names that indicate the first original natural source of the carboxylic acid. Structure of Acid Natural Source Common Name O H C OH Ants (Formica) Formic acid O CH3 C OH Vinegar (Acetum) Acetic acid O CH3CH2 C OH Basic Fat (Propio) Propionic acid O CH3CH2CH2 C OH Rancid butter (Butyrum) Butyric acid Present in aValerian herb Valeric acid O 4 CH3CH2CH2CH2CH2 C OH Goat (Caper) Caproic acid Common Names of Carboxylic Acids The common name of a carboxylic acid (R-COOH) is derived by adding the suffix –ic acid to a prefix representing the chain length of the carboxylic acid. # of Carbons Prefix Common Name of Acid 1 Form- Formic acid 2 Acet- Acetic acid 3 Propion- Propionic acid 4 Butyr- Butyric acid 5 Valer- Valeric acid 6 Capro- Caproic acid Aromatic acid Benzo- Benzoic acid 5 IUPAC Nomenclature of Aliphatic Carboxylic Acids IUPAC names of straight chain aliphatic carboxylic acids are derived by adding the suffix –oic acid to the systematic name of the parent hydrocarbon. -

Dissociation Constants of Organic Acids and Bases
DISSOCIATION CONSTANTS OF ORGANIC ACIDS AND BASES This table lists the dissociation (ionization) constants of over pKa + pKb = pKwater = 14.00 (at 25°C) 1070 organic acids, bases, and amphoteric compounds. All data apply to dilute aqueous solutions and are presented as values of Compounds are listed by molecular formula in Hill order. pKa, which is defined as the negative of the logarithm of the equi- librium constant K for the reaction a References HA H+ + A- 1. Perrin, D. D., Dissociation Constants of Organic Bases in Aqueous i.e., Solution, Butterworths, London, 1965; Supplement, 1972. 2. Serjeant, E. P., and Dempsey, B., Ionization Constants of Organic Acids + - Ka = [H ][A ]/[HA] in Aqueous Solution, Pergamon, Oxford, 1979. 3. Albert, A., “Ionization Constants of Heterocyclic Substances”, in where [H+], etc. represent the concentrations of the respective Katritzky, A. R., Ed., Physical Methods in Heterocyclic Chemistry, - species in mol/L. It follows that pKa = pH + log[HA] – log[A ], so Academic Press, New York, 1963. 4. Sober, H.A., Ed., CRC Handbook of Biochemistry, CRC Press, Boca that a solution with 50% dissociation has pH equal to the pKa of the acid. Raton, FL, 1968. 5. Perrin, D. D., Dempsey, B., and Serjeant, E. P., pK Prediction for Data for bases are presented as pK values for the conjugate acid, a a Organic Acids and Bases, Chapman and Hall, London, 1981. i.e., for the reaction 6. Albert, A., and Serjeant, E. P., The Determination of Ionization + + Constants, Third Edition, Chapman and Hall, London, 1984. BH H + B 7. Budavari, S., Ed., The Merck Index, Twelth Edition, Merck & Co., Whitehouse Station, NJ, 1996. -

Carboxylic Acids
13 Carboxylic Acids The active ingredients in these two nonprescription pain relievers are derivatives of arylpropanoic acids. See Chemical Connections 13A, “From Willow Bark to Aspirin and Beyond.” Inset: A model of (S)-ibuprofen. (Charles D. Winters) KEY QUESTIONS 13.1 What Are Carboxylic Acids? HOW TO 13.2 How Are Carboxylic Acids Named? 13.1 How to Predict the Product of a Fischer 13.3 What Are the Physical Properties of Esterification Carboxylic Acids? 13.2 How to Predict the Product of a B-Decarboxylation 13.4 What Are the Acid–Base Properties of Reaction Carboxylic Acids? 13.5 How Are Carboxyl Groups Reduced? CHEMICAL CONNECTIONS 13.6 What Is Fischer Esterification? 13A From Willow Bark to Aspirin and Beyond 13.7 What Are Acid Chlorides? 13B Esters as Flavoring Agents 13.8 What Is Decarboxylation? 13C Ketone Bodies and Diabetes CARBOXYLIC ACIDS ARE another class of organic compounds containing the carbonyl group. Their occurrence in nature is widespread, and they are important components of foodstuffs such as vinegar, butter, and vegetable oils. The most important chemical property of carboxylic acids is their acidity. Furthermore, carboxylic acids form numerous important derivatives, including es- ters, amides, anhydrides, and acid halides. In this chapter, we study carboxylic acids themselves; in Chapters 14 and 15, we study their derivatives. 457 458 CHAPTER 13 Carboxylic Acids 13.1 What Are Carboxylic Acids? Carboxyl group A J COOH The functional group of a carboxylic acid is a carboxyl group, so named because it is made group. up of a carbonyl group and a hydroxyl group (Section 1.7D). -

Experiment #10 – Properties of Carboxylic Acids and Esters
Experiment #10 – Properties of Carboxylic Acids and Esters Introduction Carboxylic acids are characterized by the carboxyl group which combines the carbonyl group of aldehydes and ketones with O the hydroxyl group of alcohols and phenols. Since the carbonyl and RCOH hydroxyl groups are directly bonded to each other each affects the properties of the other. The result is that the hydroxyl group of a A carboxylic carboxylic acid is considerably, but not completely, different from its acid. R may alcohol or phenol sibling; the same can be said when one compares be H, alkyl, the carbonyl of a carboxylic acid with that of an aldehyde or ketone. or aromatic. Just as phenols are typically 100,000 to a million times more acidic than alcohols, carboxylic acids are typically 100,000 to a million times more acidic than phenols. Nevertheless, most carboxylic acids are considered to be relatively weak acids. For example, acetic acid (R = CH3), ionizes to an extent of less than 1% when dissolved in water. When a carboxylic acid ionizes in water it donates the proton of the -OH group to a water molecule; in other words, it is a proton donor and, therefore, an acid in the Bronsted-Lowry sense. The water that accepted the proton is a base in the Bronsted-Lowry sense. The water becomes a hydronium ion and the carboxylic acid becomes a carboxylate ion in the process, as shown in the reaction below. The expression that defines the equilibrium constant for this reaction is shown below. When one realizes that in dilute aqueous solutions the concentration of water is very close to 55.5 moles of water per liter of solution, or 55.5 M, one can substitute this number for the concentration of water and come up with the acidity constant, Ka, of the acid, as shown below. -
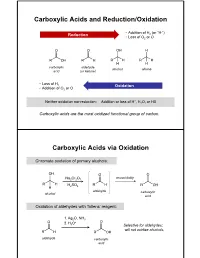
Carboxylic Acids and Reduction/Oxidation Carboxylic
Carboxylic Acids and Reduction/Oxidation • Addition of H (or “H-”) Reduction 2 • Loss of O2 or O carboxylic aldehyde alcohol alkane acid (or ketone) • Loss of H 2 Oxidation • Addition of O2 or O + Neither oxidation nor reduction: Addition or loss of H , H2O, or HX Carboxylic acids are the most oxidized functional group of carbon. Carboxylic Acids via Oxidation Chromate oxidation of primary alcohols: unavoidably Na2Cr2O7 H2SO4 aldehyde carboxylic alcohol acid Oxidation of aldehydes with Tollens’ reagent: 1. Ag2O, NH3 2. H O+ 3 Selective for aldehydes; will not oxidize alcohols. aldehyde carboxylic acid Carboxylic Acids via Oxidation Oxidative cleavage of alkynes: 1. O3 2. H2O internal alkyne Oxidation of benzylic carbons: KMnO4 Purifying Carboxylic Acids by Acid-Base Extraction Acidic and basic organic molecules can be separated from other substances by manipulating their protonation state and solubility. Let’s say we run a reaction. Na2Cr2O7 + unreacted H2SO4 + Na+ 3+ We want this… + Cr(H2O)6 + organic + HSO - …but not these. side products 4 How do we isolate our desired product from the other materials, without using distillation or chromatography? Acid-Base Extraction Step 1: Remove inorganics from organics via differential solubility. a separatory (sept) funnel - Na+ HSO4 Cr(H O) 3+ H2O 2 6 CHCl3 + organic side densities: products (H2O) = 1 g/mL (CHCl3) = 1.48 g/mL A large density difference ensures the two liquids separate completely and quickly. (Much faster than, say, oil and water.) Step 2: Add basic water to deprotonate carboxylic acid, and transfer it to the aqueous phase. - Na+ HSO4 3+ discard Cr(H2O)6 H2O layer + organic + organic side side products products add discard NaOH/ H2O CHCl3 layer + organic side products Step 3: Re-acidify carboxylate to return it to organic solution. -

Carboxylic Acids a Carbonyl with One OH Attached Is Called a Carboxylic
Carboxylic Acids A carbonyl with one OH attached is called a carboxylic acid One important property of carboxylic acids is the acidity O O pKa = 4.7 B BH H3C OH H3C O acetic acid Upon deprotonation a carboxylate is formed Nomenclature There are two important guidelines to know about carboxylic acids: 1) The carboxylic acid has the highest priority in naming 2) In common names, the point of substitution is labeled by the Greek letter counting from the carbonyl " O OH # ! This naming is common practice amongst organic chemists, e,g, substitution at α-carbon Examples (E)-2-pentenoic acid 3-bromo-2-methylpentanoic acid Or β-bromo-α-methylpentanoic acid (common) Common Names Many acid compounds have a common name Most prevalent amongst these are aromatic compounds Physical Properties Most physical properties of carboxylic acids are a result of hydrogen bonding The carboxylic acids form dimers through hydrogen bonding This hydrogen bonding causes a higher melting point and boiling point compared to compounds of similar molecular weight Acidity As observed previously, carboxylic acids are far more acidic than alcohols This is due to the stability of the anion formed after deprotonation Can also stabilize anion through inductive effects Polar bonds near anion source can stabilize negative charge (inductive effect) Consider the acetate group again: Carboxylate Salts Upon deprotonation of a carboxylic acid obtain a carboxylate salt This salt has different physical properties than the acid form (similar to the difference in amine salts discussed