Dyskeratosis Congenita Precision Panel Overview Indications Clinical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Genetics and Clinical Manifestations of Telomere Biology Disorders Sharon A
REVIEW The genetics and clinical manifestations of telomere biology disorders Sharon A. Savage, MD1, and Alison A. Bertuch, MD, PhD2 3 Abstract: Telomere biology disorders are a complex set of illnesses meric sequence is lost with each round of DNA replication. defined by the presence of very short telomeres. Individuals with classic Consequently, telomeres shorten with aging. In peripheral dyskeratosis congenita have the most severe phenotype, characterized blood leukocytes, the cells most extensively studied, the rate 4 by the triad of nail dystrophy, abnormal skin pigmentation, and oral of attrition is greatest during the first year of life. Thereafter, leukoplakia. More significantly, these individuals are at very high risk telomeres shorten more gradually. When the extent of telo- of bone marrow failure, cancer, and pulmonary fibrosis. A mutation in meric DNA loss exceeds a critical threshold, a robust anti- one of six different telomere biology genes can be identified in 50–60% proliferative signal is triggered, leading to cellular senes- of these individuals. DKC1, TERC, TERT, NOP10, and NHP2 encode cence or apoptosis. Thus, telomere attrition is thought to 1 components of telomerase or a telomerase-associated factor and TINF2, contribute to aging phenotypes. 5 a telomeric protein. Progressively shorter telomeres are inherited from With the 1985 discovery of telomerase, the enzyme that ex- generation to generation in autosomal dominant dyskeratosis congenita, tends telomeric nucleotide repeats, there has been rapid progress resulting in disease anticipation. Up to 10% of individuals with apparently both in our understanding of basic telomere biology and the con- acquired aplastic anemia or idiopathic pulmonary fibrosis also have short nection of telomere biology to human disease. -
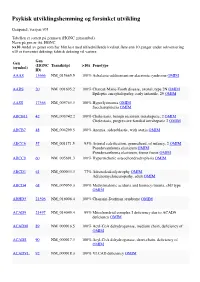
Psykisk Utviklingshemming Og Forsinket Utvikling
Psykisk utviklingshemming og forsinket utvikling Genpanel, versjon v03 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC >x10 Andel av genet som har blitt lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering x10 er forventet dekning; faktisk dekning vil variere. Gen Gen (HGNC Transkript >10x Fenotype (symbol) ID) AAAS 13666 NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AARS 20 NM_001605.2 100% Charcot-Marie-Tooth disease, axonal, type 2N OMIM Epileptic encephalopathy, early infantile, 29 OMIM AASS 17366 NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCB11 42 NM_003742.2 100% Cholestasis, benign recurrent intrahepatic, 2 OMIM Cholestasis, progressive familial intrahepatic 2 OMIM ABCB7 48 NM_004299.5 100% Anemia, sideroblastic, with ataxia OMIM ABCC6 57 NM_001171.5 93% Arterial calcification, generalized, of infancy, 2 OMIM Pseudoxanthoma elasticum OMIM Pseudoxanthoma elasticum, forme fruste OMIM ABCC9 60 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 61 NM_000033.3 77% Adrenoleukodystrophy OMIM Adrenomyeloneuropathy, adult OMIM ABCD4 68 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 21396 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 21497 NM_014049.4 99% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM 89 NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS 90 NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL 92 NM_000018.3 100% VLCAD -

Blueprint Genetics Bone Marrow Failure Syndrome Panel
Bone Marrow Failure Syndrome Panel Test code: HE0801 Is a 135 gene panel that includes assessment of non-coding variants. Is ideal for patients with a clinical suspicion of inherited bone marrow failure syndromes. The genes on this panel are included in the Comprehensive Hematology Panel. About Bone Marrow Failure Syndrome Inherited bone marrow failure syndromes (IBMFS) are a diverse set of genetic disorders characterized by the inability of the bone marrow to produce sufficient circulating blood cells. Bone marrow failure can affect all blood cell lineages causing clinical symptoms similar to aplastic anemia, or be restricted to one or two blood cell lineages. The clinical presentation may include thrombocytopenia or neutropenia. Hematological manifestations may be accompanied by physical features such as short stature and abnormal skin pigmentation in Fanconi anemia and dystrophic nails, lacy reticular pigmentation and oral leukoplakia in dyskeratosis congenita. Patients with IBMFS have an increased risk of developing cancer—either hematological or solid tumors. Early and correct disease recognition is important for management and surveillance of the diseases. Currently, accurate genetic diagnosis is essential to confirm the clinical diagnosis. The most common phenotypes that are covered by the panel are Fanconi anemia, Diamond-Blackfan anemia, dyskeratosis congenita, Shwachman-Diamond syndrome and WAS-related disorders. Availability 4 weeks Gene Set Description Genes in the Bone Marrow Failure Syndrome Panel and their clinical significance -
Copyrighted Material
1 Index Note: Page numbers in italics refer to figures, those in bold refer to tables and boxes. References are to pages within chapters, thus 58.10 is page 10 of Chapter 58. A definition 87.2 congenital ichthyoses 65.38–9 differential diagnosis 90.62 A fibres 85.1, 85.2 dermatomyositis association 88.21 discoid lupus erythematosus occupational 90.56–9 α-adrenoceptor agonists 106.8 differential diagnosis 87.5 treatment 89.41 chemical origin 130.10–12 abacavir disease course 87.5 hand eczema treatment 39.18 clinical features 90.58 drug eruptions 31.18 drug-induced 87.4 hidradenitis suppurativa management definition 90.56 HLA allele association 12.5 endocrine disorder skin signs 149.10, 92.10 differential diagnosis 90.57 hypersensitivity 119.6 149.11 keratitis–ichthyosis–deafness syndrome epidemiology 90.58 pharmacological hypersensitivity 31.10– epidemiology 87.3 treatment 65.32 investigations 90.58–9 11 familial 87.4 keratoacanthoma treatment 142.36 management 90.59 ABCA12 gene mutations 65.7 familial partial lipodystrophy neutral lipid storage disease with papular elastorrhexis differential ABCC6 gene mutations 72.27, 72.30 association 74.2 ichthyosis treatment 65.33 diagnosis 96.30 ABCC11 gene mutations 94.16 generalized 87.4 pityriasis rubra pilaris treatment 36.5, penile 111.19 abdominal wall, lymphoedema 105.20–1 genital 111.27 36.6 photodynamic therapy 22.7 ABHD5 gene mutations 65.32 HIV infection 31.12 psoriasis pomade 90.17 abrasions, sports injuries 123.16 investigations 87.5 generalized pustular 35.37 prepubertal 90.59–64 Abrikossoff -

An Update on the Biology and Management of Dyskeratosis Congenita and Related Telomere Biology Disorders
Expert Review of Hematology ISSN: 1747-4086 (Print) 1747-4094 (Online) Journal homepage: https://www.tandfonline.com/loi/ierr20 An update on the biology and management of dyskeratosis congenita and related telomere biology disorders Marena R. Niewisch & Sharon A. Savage To cite this article: Marena R. Niewisch & Sharon A. Savage (2019) An update on the biology and management of dyskeratosis congenita and related telomere biology disorders, Expert Review of Hematology, 12:12, 1037-1052, DOI: 10.1080/17474086.2019.1662720 To link to this article: https://doi.org/10.1080/17474086.2019.1662720 Accepted author version posted online: 03 Sep 2019. Published online: 10 Sep 2019. Submit your article to this journal Article views: 146 View related articles View Crossmark data Citing articles: 1 View citing articles Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=ierr20 EXPERT REVIEW OF HEMATOLOGY 2019, VOL. 12, NO. 12, 1037–1052 https://doi.org/10.1080/17474086.2019.1662720 REVIEW An update on the biology and management of dyskeratosis congenita and related telomere biology disorders Marena R. Niewisch and Sharon A. Savage Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA ABSTRACT ARTICLE HISTORY Introduction: Telomere biology disorders (TBDs) encompass a group of illnesses caused by germline Received 14 June 2019 mutations in genes regulating telomere maintenance, resulting in very short telomeres. Possible TBD Accepted 29 August 2019 manifestations range from complex multisystem disorders with onset in childhood such as dyskeratosis KEYWORDS congenita (DC), Hoyeraal-Hreidarsson syndrome, Revesz syndrome and Coats plus to adults presenting Telomere; dyskeratosis with one or two DC-related features. -

DNA Diagnostic Lab at JOHNS HOPKINS Test Requisition Part I of II
Shipping Address: DNA Diagnostic Lab at JOHNS HOPKINS 1812 Ashland Ave Sample Intake Rm 245 Test Requisition Part I of II Baltimore, MD 21205 Referrer Information Physician UPIN /NPI Genetic Counselor Contact Email: Institution Address City/State/Zip Phone Fax Additional Reports to Name /Institution Address City/State/Zip Phone Fax Patient Information *Two of these identifiers must appear on the sample *Name (Last) (First) Address City/State/Zip *Date of Birth (mm/dd/yyyy) Sex Ethnicity Position in Pedigree *Patient ID / Sample Number Sample Type Date Collected: _________________________ Venous Blood Cord Blood Cleaned Chorionic Villi Cultured Amniocytes Cultured Chorionic Villi Cultured Fibroblasts Other Culture ________________ Frozen tissue (source: _____________________) DNA (source: ______________________) PureGene Extraction Other method Reason for Test Prenatal Targeted Variant Identification Family member for Linkage analysis Carrier Presymptomatic Confirmatory/Diagnostic Diagnosis Code (ICD‐10): If the patient is pregnant: LMP Billing Information (All required documents must be received for testing to be initiated; see web site billing page for information) Credit Card – Attach Credit Card Authorization Form Check (Check # Amount of Check: $ ) Patient Insurance ‐ Contact Billing Coordinator at 443‐287‐2486 prior to submitting. Referring Center (Include address and contact person if different from that provided above) Maryland Medicaid # __________________________________ (referral required) Medicare # ____________________________________________ -

Blueprint Genetics Comprehensive Hematology and Hereditary Cancer
Comprehensive Hematology and Hereditary Cancer Panel Test code: HE1401 Is a 348 gene panel that includes assessment of non-coding variants. Is ideal for patients with a clinical suspicion of hematological disorder with genetic predisposition to malignancies. This panel is designed to detect heritable germline mutations and should not be used for the detection of somatic mutations in tumor tissue. Is not recommended for patients suspected to have anemia due to alpha-thalassemia (HBA1 or HBA2). These genes are highly homologous reducing mutation detection rate due to challenges in variant call and difficult to detect mutation profile (deletions and gene-fusions within the homologous genes tandem in the human genome). Is not recommended for patients with a suspicion of severe Hemophilia A if the common inversions are not excluded by previous testing. An intron 22 inversion of the F8 gene is identified in 43%-45% individuals with severe hemophilia A and intron 1 inversion in 2%-5% (GeneReviews NBK1404; PMID:8275087, 8490618, 29296726, 27292088, 22282501, 11756167). This test does not detect reliably these inversions. Is not recommended for patients suspected to have anemia due to alpha-thalassemia (HBA1 or HBA2). These genes are highly homologous reducing mutation detection rate due to challenges in variant call and difficult to detect mutation profile (deletions and gene-fusions within the homologous genes tandem in the human genome). About Comprehensive Hematology and Hereditary Cancer Inherited hematological diseases are a group of blood disorders with variable clinical presentation. Many of them predispose to malignancies and for example patients with inherited bone marrow failure syndromes (Fanconi anemia) have a high risk of developing cancer, either leukemia or solid tumors. -
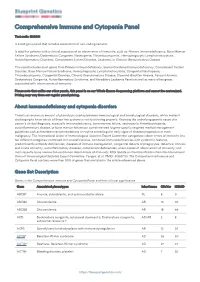
Blueprint Genetics Comprehensive Immune and Cytopenia Panel
Comprehensive Immune and Cytopenia Panel Test code: IM0901 Is a 642 gene panel that includes assessment of non-coding variants. Is ideal for patients with a clinical suspicion of an inborn error of immunity, such as, Primary Immunodeficiency, Bone Marrow Failure Syndrome, Dyskeratosis Congenita, Neutropenia, Thrombocytopenia, Hemophagocytic Lymphohistiocytosis, Autoinflammatory Disorders, Complement System Disorder, Leukemia, or Chronic Granulomatous Disease. This panel includes most genes from Primary Immunodeficiency, Severe Combined Immunodeficiency, Complement System Disorder, Bone Marrow Failure Syndrome, Hemophagocytic Lymphohistiocytosis, Congenital Neutropenia, Thrombocytopenia, Congenital Diarrhea, Chronic Granulomatous Disease, Diamond-Blackfan Anemia, Fanconi Anemia, Dyskeratosis Congenita, Autoinflammatory Syndrome, and Hereditary Leukemia Panels as well as many other genes associated with inborn errors of immunity. Please note that unlike our other panels, this panel is on our Whole Exome Sequencing platform and cannot be customized. Pricing may vary from our regular panel pricing. About immunodeficiency and cytopenia disorders There is an enormous amount of phenotypic overlap between immunological and hematological disorders, which makes it challenging to know which of these two systems is not functioning properly. Knowing the underlying genetic cause of a person’s clinical diagnosis, especially immunodeficiency, bone marrow failure, neutropenia, thrombocytopenia, autoinflammatory disease, or bone marrow failure can sometime -

WES Gene Package Oncogenetics.Xlsx
Whole Exome Sequencing Gene package Oncogenetics, version 2.1, 25‐7‐2019 Technical information DNA was enriched using Agilent SureSelect Clinical Research Exome V2 capture and paired‐end sequenced on the Illumina platform (outsourced). The aim is to obtain 8.1 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA‐MEM algorithm (reference: http://bio‐bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x A2ML1 No OMIM phenotype 610627 44 100 97 80 ACD ?Dyskeratosis congenita, autosomal dominant 6, 616553 609377 92 100 100 100 ?Dyskeratosis congenita, autosomal recessive 7, 616553 AIP Pituitary adenoma, growth hormone‐secreting, 102200 605555 78 100 100 100 Pituitary adenoma, prolactin‐secreting, -

Whole Exome Sequencing Gene Package Multiple Congenital Anomaly, Version 8.1, 31-1-2020
Whole Exome Sequencing Gene package Multiple congenital anomaly, version 8.1, 31-1-2020 Technical information DNA was enriched using Agilent SureSelect DNA + SureSelect OneSeq 300kb CNV Backbone + Human All Exon V7 capture and paired-end sequenced on the Illumina platform (outsourced). The aim is to obtain 10 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate and non-unique reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA-MEM algorithm (reference: http://bio-bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x A4GALT [Blood group, P1Pk system, P(2) phenotype], 111400 [Blood group, P1Pk system, p phenotype], 111400 NOR poly607922 146 100 100 99 AAAS Achalasia-addisonianism-alacrimia syndrome, 231550 605378 102 100 100 100 -

GENETIC TESTING REQUISITION Please Ship All NON-PRENATAL
GENETIC TESTING REQUISITION 1-844-363-4357· [email protected] Schillingallee 68 · 18057 Rostock Germany Attention Patient: Please visit your nearest LifeLabs or CML Healthcare Patient Service Centre for sample collection CONTRACT # LL: K012-01/ CML: CEN Report to Physician Billing # LifeLabs Demographic Label Ordering Physician Name Physician Signature: Ordering Physician Address: Tel: Fax: Address & Contact Info: Copy to (name & contact info): Name: Contact: Bill to Contract # K012-01 (patient does not pay at time of collection) Patient Gender: (M/F) Patient Name (Last, First): Patient DOB: (YYYY/MM/DD) Patient Address: Patient Health Card: Patient Telephone: Please ship all NON-PRENATAL samples to: LifeLabs · Attn CDS Department • 100 International Boulevard• Toronto ON• M9W6J6 TEST REQUESTED LL TR # / CML TC# □ Genetic Test - Blood Sample 2 x 4mL EDTA 4005 □ Genetic Test (Pediatric) - Blood Sample 1 x 2mL EDTA 4008 □ Genetic Test - Other Sample Type 4014 PRENATAL SAMPLES: Please ship directly to CENTOGENE. Date Blood Collected (YYYY/MM/DD): ___________ Time Blood Collected (HH:MM)) :________ Collector Name: - ___________________ GENETIC TESTING CONSENT I understand that a DNA specimen will be sent to LifeLabs for genetic testing. My physician has told me about the condition(s) being tested and its genetic basis. I am aware that correct information about the relationships between my family members is important. I agree that my specimen and personal health information may be sent to Centogene AG at their lab in Germany (address below). To ensure accurate testing, I agree that the results of any genetic testing that I have had previously completed by Centogene AG may be shared with LifeLabs. -

Detailed Listing of Compassionate Allowance Cases
DETAILED LISTING OF COMPASSIONATE ALLOWANCE CASES ACUTE LEUKEMIA Acute Lymphoblastic Leukemia, Acute Myeloid Leukemia Adrenal Carcinoma, Adrenocortical Carcinoma, Adrenocortical ADRENAL CANCER Cancer, Cancer of the Adrenal Cortex, Carcinoma of the Adrenal Cortex Lymphoma - non-Hodgkin's; Lymphocytic lymphoma; ADULT NON-HODGKIN Histiocytic lymphoma; Lymphoblastic lymphoma; Cancer - non- LYMPHOMA Hodgkin's lymphoma ADULT ONSET HUNTINGTON Huntington's chorea; Huntington's Disease DISEASE AGS; Cree Encephalitis; Encephalopathy with Basal Ganglia Calcification; Pseudo-TORCH Syndrome; AICARDI - GOUTIERES Pseudotoxoplasmosis Syndrome; Familial Infantile SYNDROME Encephalopathy with Intracranial Calcification and Chronic Cerebrospinal Fluid Lymphocytosis Alexander Syndrome, Dysmyelogenic Leukodystrophy, Dysmyelogenic Leukodystrophy-Megalobare, Fribrinoid ALEXANDER DISEASE (ALX) - Degeneration of Astrocytes-Infantile type, Fibrinoid NEONATAL AND INFANTILE Leukodystrophy-Infantile type, Hyaline Panneuropathy, Leukodystrophy with Rosenthal Fibers, Megalencephaly with Hyaline Inclusion, Megalencephaly with Hyaline Panneuropathy Allan-Herndon Syndrome; X-linked Intellectual Deficit with ALLAN-HERNDON-DUDLEY Hypotonia; Monocarboxylate Transporter 8 Deficiency; MCT8 SYNDROME Deficiency; MCT8 Specific Thyroid Hormone Cell Transporter Deficiency; MCT8 SLC16A2 Holoprosencephaly; HPE; Holoprosencephaly 1 Alobar; ALOBAR Familial Alobar Holoprosencephaly; Holoprosencephaly HOLOPROSENCEPHALY Sequence Alpers Syndrome; Alpers Progressive Infantile Poliodystrophy;