Effective July 1, 2011
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

The Inhaled Steroid Ciclesonide Blocks SARS-Cov-2 RNA Replication by Targeting Viral
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting viral 2 replication-transcription complex in culture cells 3 4 Shutoku Matsuyamaa#, Miyuki Kawasea, Naganori Naoa, Kazuya Shiratoa, Makoto Ujikeb, Wataru 5 Kamitanic, Masayuki Shimojimad, and Shuetsu Fukushid 6 7 aDepartment of Virology III, National Institute of Infectious Diseases, Tokyo, Japan 8 bFaculty of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan 9 cDepartment of Infectious Diseases and Host Defense, Gunma University Graduate School of 10 Medicine, Gunma, Japan 11 dDepartment of Virology I, National Institute of Infectious Diseases, Tokyo, Japan. 12 13 Running Head: Ciclesonide blocks SARS-CoV-2 replication 14 15 #Address correspondence to Shutoku Matsuyama, [email protected] 16 17 Word count: Abstract 149, Text 3,016 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 18 Abstract 19 We screened steroid compounds to obtain a drug expected to block host inflammatory responses and 20 MERS-CoV replication. Ciclesonide, an inhaled corticosteroid, suppressed replication of MERS-CoV 21 and other coronaviruses, including SARS-CoV-2, the cause of COVID-19, in cultured cells. The 22 effective concentration (EC90) of ciclesonide for SARS-CoV-2 in differentiated human bronchial 23 tracheal epithelial cells was 0.55 μM. -
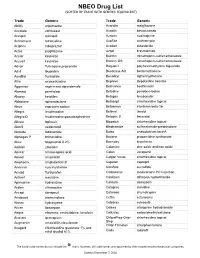
Drug List (SORTED by TRADE with GENERIC EQUIVALENT)
NBEO Drug List (SORTED BY TRADE WITH GENERIC EQUIVALENT) Trade Generic Trade Generic Abilify aripiprazole Avandia rosiglitazone Accolate zafirlukast Avastin bevacizumab Accupril quinapril Azasan azathioprine Achromycin tetracycline AzaSite azithromycin Aciphex rabeprazole Avodart dutasteride Actos pioglitazone Azopt brinzolamide Acular ketorolac Bactrim trimethoprim-sulfamethoxazole Acuvail ketorolac Bactrim DS trimethoprim-sulfamethoxazole Advair fluticasone propionate Baquacil polyhexamethylene biguanide Advil ibuprofen Beconase AQ beclomethasone AeroBid flunisolide Benadryl diphenhydramine Afrin oxymetazoline Bepreve Bepotastine besilate Aggrenox aspirin and dipyridamole Besivance besifloxacin Alamast pemirolast Betadine povidone-iodine Alaway ketotifen Betagan levobunolol Aldactone spironolactone Betasept chlorhexidine topical Aleve naproxen sodium Betaseron interferon beta-1b Allegra fexofenadine Betimol timolol Allegra-D fexofenadine-pseudoephedrine Betoptic S betaxolol Alluvia lopinavir Biopatch chlorhexidine topical Alocril nedocromil Blephamide sulfacetamide-prednisolone Alomide lodoxamide Botox onabotulinum toxinA Alphagan P brimonidine Brolene propamidine isethionate Alrex loteprednol 0.2% Bromday bromfenac Ambien zolpidem Calamine zinc oxide and iron oxide Amicar aminocaproic acid Calan verapamil Amoxil amoxicillin Calgon Vesta chlorhexidine topical Amphocin amphotericin B Capoten captopril Anectine succinylcholine Carafate sucralfate Ansaid flurbiprofen Carbocaine mepivacaine HCl injection Antivert meclizine Cardizem diltiazem -

Sir, Mistaken Eye Drops and Subsequent Instillation of Superglue
Sir, in susceptible (steroid-responsive) patients. Rimexolone Mistaken eye drops and subsequent instillation of 1 % ophthalmic suspension is a recently developed superglue topical corticosteroid with effective anti-inflammatory z 3 A 60-year-old man presented himself to the casualty properties as well as a reduced risk of increased IOP. , department after accidentally instilling superglue into We present a case report of a patient with markedly his eyes. He had traditionally put eye drops in himself in elevated lOP associated with the use of 1% rimexolone the evening. On this occasion he had mistaken his wife's suspension. fingernail glue for the eye drops as it stood on the bedside cabinet. The bottles were very similar in reduced Case report light, as both were a dropper design for delivery and the same compact size. A 52-year-old woman with a history of toxic epidermal Once the glue, of which the major constituent was necrolysis has been attending our institution since 1992. cyanoacrylate, contacted his eye it caused immense As a result of her condition she developed dry eyes sudden pain causing him to close his eye more. The glue which required punctal occlusion and eye lubricants, then set quickly and thus he presented with a including autologous serum drops. She had marked permanently closed eye. The upper lid was adherent to keratinisation of the tarsal conjunctiva especially in the the cornea, as when the eye movements were tested the left eye, which necessitated mucous membrane grafting. lid moved. Her condition was further complicated by metaplastic He was followed up and after two consultations he eyelashes, which were treated with cryotherapy. -

General Agreement on Tariffs Andtrade
RESTRICTED GENERAL AGREEMENT TAR/W/87/Rev.1 16 June 1994 ON TARIFFS AND TRADE Limited Distribution (94-1266) Committee on Tariff Concessions HARMONIZED COMMODITY DESCRIPTION AND CODING SYSTEM (Harmonized System) Classification of INN Substances Revision The following communication has been received from the Nomenclature and Classification Directorate of the Customs Co-operation Council in Brussels. On 25 May 1993, we sent you a list of the INN substances whose classification had been discussed and decided by the Harmonized System Committee. At the time, we informed you that the classification of two substances, clobenoside and meclofenoxate, would be decided later. Furthermore, for some of the chemicals given in that list, one of the contracting parties had entered a reservation and the Harmonized System Committee therefore reconsidered its earlier decision in those cases. I am therefore sending you herewith a revised complete list of the classification decisions of the INN substances. In this revised list, two substances have been added and the classifications of two have been revised as explained below: (a) Addition Classification of clobenoside, (subheading 2940.00) and meclofenoxate (subheading 2922.19). (b) Amendment Etafedrine and moxidentin have now been reclassified in subheadings 2939.40 and 2932.29 respectively. The list of INN substances reproduced hereafter is available only in English. TAR/W/87/Rev. 1 Page 2 Classification of INN Substances Agreed by the Harmonized System Committee in April 1993 Revision Description HS Code -

Understanding Pharmacology for Pharmacy Technicians 777
Index Note: Key Terms and Defi nitions are denoted by the boldfaced page numbers. 5-alpha dihydrotestosterone, 196 acromegaly, 129, 132–34, 144–45t afterload, 318 5-alpha reductase inhibitors, 214, 224t Actemra (tocilizumab), 263t age-related macular degeneration, 740 IND 5-ASA. See Mesalamine ACTH. See Adrenocorticotropic hormone (ACTH) Aggrastat (tirofi ban), 315, 338t 5-HT serotonin antagonists. See Serotonin antagonists ACTH (cosyntropin) stimulation test, 159–60 agonist-antagonists, 58, 61t 5-hydroxytryptamine (5-HT). See Serotonin ActHIB (haemophilus B vaccine), 644t agonists, 49 12-step support group, 115 action potential, 45, 47–48 alpha, 739 17ß estradiol, 220t, 695t Actiq (fentanyl), 61t beta2, 381–82, 390 Activase (alteplase), 67, 338t central alpha2, 297, 304t, 307t activated partial thromboplastin time (aPTT), 539, 553 dopamine, 69, 84t, 109, 132–33, 144t, 305t A active immunity, 634–35 general, 31 A and D Original (vitamin A and D), 725t Activella (estradiol/norethindrone acetate), 221t long-acting beta2, 381, 382, 384, 386, 390 AA. See Arachidonic acid (AA) Actonel (risedronate), 258t opiate, 54–57, 57t AABP Allergen Ear Drops, 761t ActoPlus Met (pioglitazone/metformin), 185, 189t opiate partial, 58 abacavir, 608, 617t Actos (pioglitazone), 184, 190t selective, 31–32 abacavir/lamivudine combinations, 619t Acular (ketorolac), 749t selective serotonin, 65, 66 abarelix, 666, 670t Acular LS (ketorolac), 749t short-acting beta2, 381–82, 384 abatacept, 253, 263t acupressure/acupuncture, 431 agranulocytosis, 109 abciximab, 315, 338t, 549 acute coronary syndromes, 309, 314–18, 336–40t A-Hydrocort (hydrocortisone), 164t Abelcet (amphotericin B lipid), 624, 630t Acuvail (ketorolac), 749t AIDS. See Acquired immunodefi ciency syndrome ABG. -

Contact Dermatitis to Medications and Skin Products
Clinical Reviews in Allergy & Immunology (2019) 56:41–59 https://doi.org/10.1007/s12016-018-8705-0 Contact Dermatitis to Medications and Skin Products Henry L. Nguyen1 & James A. Yiannias2 Published online: 25 August 2018 # Springer Science+Business Media, LLC, part of Springer Nature 2018 Abstract Consumer products and topical medications today contain many allergens that can cause a reaction on the skin known as allergic contact dermatitis. This review looks at various allergens in these products and reports current allergic contact dermatitis incidence and trends in North America, Europe, and Asia. First, medication contact allergy to corticosteroids will be discussed along with its five structural classes (A, B, C, D1, D2) and their steroid test compounds (tixocortol-21-pivalate, triamcinolone acetonide, budesonide, clobetasol-17-propionate, hydrocortisone-17-butyrate). Cross-reactivities between the steroid classes will also be examined. Next, estrogen and testosterone transdermal therapeutic systems, local anesthetic (benzocaine, lidocaine, pramoxine, dyclonine) antihistamines (piperazine, ethanolamine, propylamine, phenothiazine, piperidine, and pyrrolidine), top- ical antibiotics (neomycin, spectinomycin, bacitracin, mupirocin), and sunscreen are evaluated for their potential to cause contact dermatitis and cross-reactivities. Finally, we examine the ingredients in the excipients of these products, such as the formaldehyde releasers (quaternium-15, 2-bromo-2-nitropropane-1,3 diol, diazolidinyl urea, imidazolidinyl urea, DMDM hydantoin), the non- formaldehyde releasers (isothiazolinones, parabens, methyldibromo glutaronitrile, iodopropynyl butylcarbamate, and thimero- sal), fragrance mixes, and Myroxylon pereirae (Balsam of Peru) for contact allergy incidence and prevalence. Furthermore, strategies, recommendations, and two online tools (SkinSAFE and the Contact Allergen Management Program) on how to avoid these allergens in commercial skin care products will be discussed at the end. -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational -

Similarities Between Corticosteroids
The Science Journal of the Lander College of Arts and Sciences Volume 7 Number 2 Spring 2014 - 1-1-2014 Similarities Between Corticosteroids M. Einhorn Touro College Follow this and additional works at: https://touroscholar.touro.edu/sjlcas Part of the Chemical and Pharmacologic Phenomena Commons Recommended Citation Einhorn, M. (2014). Similarities Between Corticosteroids. The Science Journal of the Lander College of Arts and Sciences, 7(2). Retrieved from https://touroscholar.touro.edu/sjlcas/vol7/iss2/6 This Article is brought to you for free and open access by the Lander College of Arts and Sciences at Touro Scholar. It has been accepted for inclusion in The Science Journal of the Lander College of Arts and Sciences by an authorized editor of Touro Scholar. For more information, please contact [email protected]. Similarities Between Corticosteroids documented, and medical practitioners administer these ments fluctuates depending on the strength of the cortico- Similarities Between Corticosteroids drugs because the effects are beneficial to the patients. steroid. M. Einhorn Corticosteroids are sometimes referred to as a “miracle In a double blind study testing the bioavailability of drug” because of the diverse pathologies that are alleviat- different corticosteroids, desoximetasone 0.25%, clobeta- ed or managed by these drugs. The precise mechanism of Abstract sol-17-propionate .05%, hydrocortisone 1.0%, and betame- action, nevertheless, is as of yet unknown. Much research thasone dipropionate .064% were applied on the volar Corticosteroids are a class of potent drugs with important physiological effects on the body. Regular use is linked with has been done to discover this mechanism, and varying forearm of 30 healthy volunteers. -

Ocular-Hypertensive and Anti-Inflammatory Response to Rimexolone Therapy in Children
CLINICAL SCIENCES Ocular-Hypertensive and Anti-inflammatory Response to Rimexolone Therapy in Children Dorothy S. P. Fan, FRCS; Christopher B. O. Yu, FRCOphth; Thomas Y. H. Chiu, MRCS; Chun Yu Wong, FRCOphthHK, FRCS; Joan S. K. Ng, FRCS; Chi Pui Pang, DPhil; Dennis S. C. Lam, FRCS, FRCOphth Objective: To compare the ocular-hypertensive and anti- study. Intraocular pressure increased significantly in inflammatory response to rimexolone (116-hydroxy- both treatment groups compared with the preoperative 16␣fluoro-6␣methylpresdnisolone) and fluorometho- values (PϽ.001). The mean (SD) peak intraocular pres- lone (21-deoxy-9␣fluoro-6␣methylprednisolone) therapy sure was significantly higher in the rimexolone-treated in children’s eyes. group, 19.7 (6.1) vs 17.6 (4.6) mm Hg (PϽ.001). Simi- larly, the mean (SD) net increase in intraocular pressure Methods: With parental consent, children who under- (PϽ.001), was also higher in the rimexolone-treated went surgical procedures for bilateral symmetric strabis- eyes, 5.9 (4.4) vs 3.9 (4.1) mm Hg (PϽ.001). In addi- mus from January 18, 2000, through November 16, 2001, tion, a greater percentage of the rimexolone-treated were recruited. One eye was randomized to receive topi- patients had no conjunctival erythema on days 13 cal 1% rimexolone while the contralateral eye received (11.1% vs 0.0%) and 20 (88.9% vs 55.6%) (P=.03). topical 0.1% fluorometholone, 4 times daily for 4 weeks. Conclusions: Rimexolone seems to be a more effective Main Outcome Measures: Intraocular pressures and anti-inflammatory agent than fluorometholone. How- anti-inflammatory responses were the main outcome mea- ever, unlike adults, the ocular-hypertensive effect in chil- sures and were serially measured postoperatively for 8 dren treated with rimexolone was higher. -

Therapeutic Drug Class
EFFECTIVE Version Department of Vermont Health Access Updated: 02/16/17 Pharmacy Benefit Management Program /2016 Vermont Preferred Drug List and Drugs Requiring Prior Authorization (includes clinical criteria) The Commissioner for Office of Vermont Health Access shall establish a pharmacy best practices and cost control program designed to reduce the cost of providing prescription drugs, while maintaining high quality in prescription drug therapies. The program shall include: "A preferred list of covered prescription drugs that identifies preferred choices within therapeutic classes for particular diseases and conditions, including generic alternatives" From Act 127 passed in 2002 The following pages contain: • The therapeutic classes of drugs subject to the Preferred Drug List, the drugs within those categories and the criteria required for Prior Authorization (P.A.) of non-preferred drugs in those categories. • The therapeutic classes of drugs which have clinical criteria for Prior Authorization may or may not be subject to a preferred agent. • Within both of these categories there may be drugs or even drug classes that are subject to Quantity Limit Parameters. Therapeutic class criteria are listed alphabetically. Within each category the Preferred Drugs are noted in the left-hand columns. Representative non- preferred agents have been included and are listed in the right-hand column. Any drug not listed as preferred in any of the included categories requires Prior Authorization. GHS/Change Healthcare GHS/Change Healthcare GHS/Change Healthcare Sr. Account Manager: PRESCRIBER Call Center: PHARMACY Call Center: PA Requests PA Requests Michael Ouellette, RPh Tel: 1-844-679-5363; Fax: 1-844-679-5366 Tel: 1-844-679-5362 Tel: 802-922-9614 Note: Fax requests are responded to within 24 hrs.