Understanding Pharmacology for Pharmacy Technicians 777
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Fluorometholone Ophthalmic Suspension 0.1% W/V Corticosteroid Anti-Inflammatory
PRODUCT MONOGRAPH PrFML® Fluorometholone Ophthalmic Suspension 0.1% w/v Corticosteroid Anti-Inflammatory Allergan Inc. Date of Preparation: Markham, ON October 30, 1972 L6G 0B5 Date of Revision: May 2, 2018 Submission Control No: 214474 Page 1 of 12 NAME OF DRUG Pr ® FML Fluorometholone Ophthalmic Suspension 0.1% w/v THERAPEUTIC CLASSIFICATION Topical corticosteroid ACTIONS Corticosteroids inhibit the inflammatory response to a variety of inciting agents of a mechanical, chemical and immunological nature. They inhibit edema, fibrin deposition, capillary dilation, leukocyte migration, phagocytic activity, capillary proliferation, fibroblast proliferation, deposition of collagen and scar formation associated with inflammation. Corticosteroids are thought to act by controlling the rate of synthesis of proteins. Corticosteroids and their derivatives are capable of producing a rise in intraocular pressure. INDICATIONS FML® (fluorometholone ophthalmic suspension 0.1% w/v) is indicated for the treatment of steroid- responsive inflammation of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe. CONTRAINDICATIONS FML® is contraindicated in: Superficial (or epithelial) herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, and other viral diseases of the cornea and conjunctiva. Fungal diseases of ocular structures. Mycobacterial infections of the eye (e.g., Tuberculosis of the eye). Acute untreated infections of the eye. Hypersensitivity to the constituents of this medication (for a listing of ingredients, see PHARMACEUTICAL INFORMATION), or hypersensitivity to other corticosteroids. Page 2 of 12 WARNINGS Use of topical corticosteroids may cause increased intraocular pressure (IOP) in certain individuals. It is necessary that the IOP be checked frequently in patients with a history of glaucoma. Use of corticosteroids may prolong the course and may exacerbate the severity of many viral eye infections (including herpes simplex). -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Penetration of Synthetic Corticosteroids Into Human Aqueous Humour
Eye (1990) 4, 526--530 Penetration of Synthetic Corticosteroids into Human Aqueous Humour C. N. 1. McGHEE,1.3 D. G. WATSON, 3 1. M. MIDGLEY, 3 M. 1. NOBLE, 2 G. N. DUTTON, z A. I. FERNl Glasgow Summary The penetration of prednisolone acetate (1%) and fluorometholone alcohol (0.1%) into human aqueous humour following topical application was determined using the very sensitive and specific technique of Gas Chromatography with Mass Spec trometry (GCMS). Prednisolone acetate afforded peak mean concentrations of 669.9 ng/ml within two hours and levels of 28.6 ng/ml in aqueous humour were detected almost 24 hours post application. The peak aqueous humour level of flu orometholone was S.lng/ml. The results are compared and contrasted with the absorption of dexamethasone alcohol (0.1%), betamethasone sodium phosphate (0.1 %) and prednisolone sodium phosphate (0.5%) into human aqueous humour. Topical corticosteroid preparations have been prednisolone acetate (1.0%) and fluorometh used widely in ophthalmology since the early alone alcohol (0.1 %) (preliminary results) 1960s and over the last 10 years the choice of into the aqueous humour of patients under preparations has become larger and more going elective cataract surgery. varied. Unfortunately, data on the intraocular penetration of these steroids in humans has SUbjects and Methods not paralleled the expansion in the number of Patients who were scheduled to undergo rou available preparations; indeed until recently, tine cataract surgery were recruited to the estimation of intraocular penetration has study and informed consent was obtained in been reliant upon extrapolation of data from all cases (n=88), Patients with corneal disease animal models (see Watson et ai., 1988, for or inflammatory ocular conditions which bibliography). -

Electronic Supplementary Information: Solid-Phase Extraction As Sample Preparation of Water Samples for Cell-Based and Other in Vitro Bioassays
Electronic Supplementary Material (ESI) for Environmental Science: Processes & Impacts. This journal is © The Royal Society of Chemistry 2018 Electronic Supplementary Information: Solid-phase extraction as sample preparation of water samples for cell-based and other in vitro bioassays Peta A. Neale, Werner Brack, Selim Aїt-Aїssa, Wibke Busch, Juliane Hollender, Martin Krauss, Emmanuelle Maillot-Maréchal, Nicole A. Munz, Rita Schlichting, Tobias Schulze, Bernadette Vogler, Beate I. Escher Table S1: Studies that have determined the BEQbio,extract/BEQchem,extract ratio to assess chemical SPE recovery expressed as effect in a water matrix. Table S2: Studies that have determined the BEQbio,extract/BEQchem,nominal ratio to assess chemical SPE recovery expressed as effect in a water matrix. Table S3: List of the 579 chemicals spiked into Wormsgraben water (provided in Excel file). Section S1: Chemical analysis of LVSPE recovery experiment. Section S2: Chemical analysis of multi-layer SPE recovery experiments. Table S4: Measured concentration in ng/L in the water extract and water + mix extract, as well as the nominal spiked concentration in ng/L and the fraction of each chemical recovered by LVSPE 1 (frecovery,i). frecovery,i is also provided for Schulze et al. (provided in Excel file). Table S5: Results for background, recovery and calibration samples for calculation of frecovery for multi-layer SPE cartridges (provided in Excel file). Figure S1: Ratio of frecovery,i for LVSPE from the current study and frecovery,i for LVSPE (HR-X only) 1 from Schulze et al. and ratio of frecovery,i for LVSPE and frecovery,i for multi-layer SPE cartridges (both measured in current study). -

The Inhaled Steroid Ciclesonide Blocks SARS-Cov-2 RNA Replication by Targeting Viral
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting viral 2 replication-transcription complex in culture cells 3 4 Shutoku Matsuyamaa#, Miyuki Kawasea, Naganori Naoa, Kazuya Shiratoa, Makoto Ujikeb, Wataru 5 Kamitanic, Masayuki Shimojimad, and Shuetsu Fukushid 6 7 aDepartment of Virology III, National Institute of Infectious Diseases, Tokyo, Japan 8 bFaculty of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan 9 cDepartment of Infectious Diseases and Host Defense, Gunma University Graduate School of 10 Medicine, Gunma, Japan 11 dDepartment of Virology I, National Institute of Infectious Diseases, Tokyo, Japan. 12 13 Running Head: Ciclesonide blocks SARS-CoV-2 replication 14 15 #Address correspondence to Shutoku Matsuyama, [email protected] 16 17 Word count: Abstract 149, Text 3,016 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 18 Abstract 19 We screened steroid compounds to obtain a drug expected to block host inflammatory responses and 20 MERS-CoV replication. Ciclesonide, an inhaled corticosteroid, suppressed replication of MERS-CoV 21 and other coronaviruses, including SARS-CoV-2, the cause of COVID-19, in cultured cells. The 22 effective concentration (EC90) of ciclesonide for SARS-CoV-2 in differentiated human bronchial 23 tracheal epithelial cells was 0.55 μM. -

Effective July 1, 2011
MISSISSIPPI DIVISION OF MEDICAID PREFERRED DRUG LIST Effective July 1, 2011 BODY SYSTEM THERAPEUTIC CLASS PREFERRED AGENTS NON-PREFERRED AGENTS NOTES ANALGESICS ANALGESICS, fentanyl patches AVINZA (morphine) NARCOTIC-LONG-ACTING KADIAN (morphine) BUTRANS (buprenorphine) methadone DURAGESIC (fentanyl) morphine ER EMBEDA (morphine/naltrexone) EXALGO (hydromorphone) OPANA ER (oxymorphone) oxycodone ER OXYCONTIN (oxycodone) RYZOLT (tramadol) ULTRAM ER (tramadol) ANALGESICS, NARCOTIC- acetaminophen/codeine ABSTRAL (fentanyl) SHORT-ACTING aspirin/codeine butalbital/APAP/caffeine/codeine codeine butalbital/ASA/caffeine/codeine dihydrocodeine/ APAP/caffeine DARVON-N (propoxyphene) hydrocodone/APAP DILAUDID liquid (hydromorphone) hydrocodone/ibuprofen fentanyl hydromorphone FENTORA (fentanyl) IBUDONE (hydrocodone/ibuprofen) levorphanol meperidine NUCYNTA (tapentadol) morphine ONSOLIS (fentanyl) oxycodone OPANA (oxymorphone) oxycodone/APAP pentazocine/naloxone oxycodone/aspirin propoxyphene oxycodone/ibuprofen propoxyphene/APAP pentazocine/APAP REPREXAIN tramadol (hydrocodone/ibuprofen) tramadol/APAP RYBIX (tramadol) VIMOVO (naproxen/esomeprazole) ZAMICET (hydrocodone/APAP) ZOLVIT (hydrocodone/APAP) ANALGESICS/ANESTHETICS, FLECTOR (diclofenac epolamine) PENNSAID Solution TOPICAL LIDODERM (lidocaine) (diclofenac sodium ) VOLTAREN Gel (diclofenac sodium) ANTIHYPERURICEMICS allopurinol ULORIC (febuxostat) COLCRYS (colchicine) probenecid probenecid/colchicine ANALGESICS (continued) Unless otherwise stated, the listing of a particular brand or generic -

New Zealand Data Sheet
NEW ZEALAND DATA SHEET 1. PRODUCT NAME FML® 0.1% w/v eye drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Fluorometholone 1 mg/mL (0.1% w/v) For the full list of excipients, see Section 6.1. 3. PHARMACEUTICAL FORM A topical anti-inflammatory glucosteroid ophthalmic suspension. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications For steroid responsive inflammation of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe. 4.2 Dose and method of administration Bottle should be shaken before use. 1 to 2 drops instilled into the conjunctival sac two to four times daily. During the initial 24 to 48 hours, the dosage may be safely increased to 2 drops every hour. Care should be taken not to discontinue therapy prematurely. In chronic conditions, withdrawal of treatment should be carried out by gradually decreasing the frequency of applications. In order to minimise systemic absorption of FML® eye drops, apply pressure to the tear duct immediately following administration of the drug. Use in children: Safety and effectiveness have not been demonstrated in children under 2 years of age. 4.3 Contraindications FML® is contraindicated in patients with: acute superficial (or epithelial) Herpes simplex keratitis (dendritic keratitis), fungal diseases of ocular structures, vaccinia, varicella, mycobacterial infection of the eye and most other viral diseases of the cornea and conjunctiva, tuberculosis of the eye, hypersensitivity to the active substance or to any of the excipients listed in section 6.1. FML® (fluorometholone) 0.1% w/v eye drops Data sheet Version 5.0 Page 1 of 6 4.4 Special warnings and precautions for use Steroid medication in the treatment of patients with a history of Herpes simplex keratitis requires great caution. -
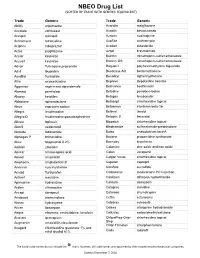
Drug List (SORTED by TRADE with GENERIC EQUIVALENT)
NBEO Drug List (SORTED BY TRADE WITH GENERIC EQUIVALENT) Trade Generic Trade Generic Abilify aripiprazole Avandia rosiglitazone Accolate zafirlukast Avastin bevacizumab Accupril quinapril Azasan azathioprine Achromycin tetracycline AzaSite azithromycin Aciphex rabeprazole Avodart dutasteride Actos pioglitazone Azopt brinzolamide Acular ketorolac Bactrim trimethoprim-sulfamethoxazole Acuvail ketorolac Bactrim DS trimethoprim-sulfamethoxazole Advair fluticasone propionate Baquacil polyhexamethylene biguanide Advil ibuprofen Beconase AQ beclomethasone AeroBid flunisolide Benadryl diphenhydramine Afrin oxymetazoline Bepreve Bepotastine besilate Aggrenox aspirin and dipyridamole Besivance besifloxacin Alamast pemirolast Betadine povidone-iodine Alaway ketotifen Betagan levobunolol Aldactone spironolactone Betasept chlorhexidine topical Aleve naproxen sodium Betaseron interferon beta-1b Allegra fexofenadine Betimol timolol Allegra-D fexofenadine-pseudoephedrine Betoptic S betaxolol Alluvia lopinavir Biopatch chlorhexidine topical Alocril nedocromil Blephamide sulfacetamide-prednisolone Alomide lodoxamide Botox onabotulinum toxinA Alphagan P brimonidine Brolene propamidine isethionate Alrex loteprednol 0.2% Bromday bromfenac Ambien zolpidem Calamine zinc oxide and iron oxide Amicar aminocaproic acid Calan verapamil Amoxil amoxicillin Calgon Vesta chlorhexidine topical Amphocin amphotericin B Capoten captopril Anectine succinylcholine Carafate sucralfate Ansaid flurbiprofen Carbocaine mepivacaine HCl injection Antivert meclizine Cardizem diltiazem -

Ophthalmic Anti-Inflammatories Review 06/10/2010
Ophthalmic Anti-Inflammatories Review 06/10/2010 Copyright © 2004 - 2010 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage and retrieval system without the express written consent of Provider Synergies, L.L.C. All requests for permission should be mailed to: Attention: Copyright Administrator Intellectual Property Department Provider Synergies, L.L.C. 10101 Alliance Road, Ste 201 Cincinnati, Ohio 45242 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is -

Sir, Mistaken Eye Drops and Subsequent Instillation of Superglue
Sir, in susceptible (steroid-responsive) patients. Rimexolone Mistaken eye drops and subsequent instillation of 1 % ophthalmic suspension is a recently developed superglue topical corticosteroid with effective anti-inflammatory z 3 A 60-year-old man presented himself to the casualty properties as well as a reduced risk of increased IOP. , department after accidentally instilling superglue into We present a case report of a patient with markedly his eyes. He had traditionally put eye drops in himself in elevated lOP associated with the use of 1% rimexolone the evening. On this occasion he had mistaken his wife's suspension. fingernail glue for the eye drops as it stood on the bedside cabinet. The bottles were very similar in reduced Case report light, as both were a dropper design for delivery and the same compact size. A 52-year-old woman with a history of toxic epidermal Once the glue, of which the major constituent was necrolysis has been attending our institution since 1992. cyanoacrylate, contacted his eye it caused immense As a result of her condition she developed dry eyes sudden pain causing him to close his eye more. The glue which required punctal occlusion and eye lubricants, then set quickly and thus he presented with a including autologous serum drops. She had marked permanently closed eye. The upper lid was adherent to keratinisation of the tarsal conjunctiva especially in the the cornea, as when the eye movements were tested the left eye, which necessitated mucous membrane grafting. lid moved. Her condition was further complicated by metaplastic He was followed up and after two consultations he eyelashes, which were treated with cryotherapy. -

USP Reference Standards Catalog
Last Updated On: January 6, 2016 USP Reference Standards Catalog Catalog # Description Current Lot Previous Lot CAS # NDC # Unit Price Special Restriction 1000408 Abacavir Sulfate R028L0 F1L487 (12/16) 188062-50-2 $222.00 (200 mg) 1000419 Abacavir Sulfate F0G248 188062-50-2 $692.00 Racemic (20 mg) (4-[2-amino-6-(cyclo propylamino)-9H-pur in-9yl]-2-cyclopenten e-1-methanol sulfate (2:1)) 1000420 Abacavir Related F1L311 F0H284 (10/13) 124752-25-6 $692.00 Compound A (20 mg) ([4-(2,6-diamino-9H- purin-9-yl)cyclopent- 2-enyl]methanol) 1000437 Abacavir Related F0M143 N/A $692.00 Compound D (20 mg) (N6-Cyclopropyl-9-{( 1R,4S)-4-[(2,5-diami no-6-chlorpyrimidin- 4-yloxy)methyl] cyclopent-2-enyl}-9H -purine-2,6-diamine) 1000441 Abacavir Related F1L318 F0H283 (10/13) N/A $692.00 Compound B (20 mg) ([4-(2,5-diamino-6-c Page 1 Last Updated On: January 6, 2016 USP Reference Standards Catalog Catalog # Description Current Lot Previous Lot CAS # NDC # Unit Price Special Restriction hloropyrimidin-4-yla mino)cyclopent-2-en yl]methanol) 1000452 Abacavir Related F1L322 F0H285 (09/13) 172015-79-1 $692.00 Compound C (20 mg) ([(1S,4R)-4-(2-amino -6-chloro-9H-purin-9 -yl)cyclopent-2-enyl] methanol hydrochloride) 1000485 Abacavir Related R039P0 F0J094 (11/16) N/A $692.00 Compounds Mixture (15 mg) 1000496 Abacavir F0J102 N/A $692.00 Stereoisomers Mixture (15 mg) 1000500 Abacavir System F0J097 N/A $692.00 Suitability Mixture (15 mg) 1000521 Acarbose (200 mg) F0M160 56180-94-0 $222.00 (COLD SHIPMENT REQUIRED) 1000532 Acarbose System F0L204 N/A $692.00 Suitability