MD-RES-10-2017-000550 Revised 171120
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Betamethasone
Betamethasone Background Betamethasone is a potent, long-acting, synthetic glucocorticoid widely used in equine veterinary medicine as a steroidal anti-inflammatory.1 It is often administered intra-articularly for control of pain associated with inflammation and osteoarthritis.2 Betamethasone is a prescription medication and can only be dispensed from or upon the request of a http://en.wikipedia.org/wiki/Betamethasone#/media/File:Betamethasone veterinarian. It is commercially available .png in a variety of formulations including BetaVet™, BetaVet Soluspan Suspension® and Betasone Aqueous Suspension™.3 Betamethasone can be used intra-articularly, intramuscularly, by inhalation, and topically.4 When administered intra-articularly, it is often combined with other substances such as hyaluronan.5 Intra-articular and intramuscular dosages range widely based upon articular space, medication combination protocol, and practitioner preference. Betamethasone is a glucocorticoid receptor agonist which binds to various glucocorticoid receptors setting off a sequence of events affecting gene transcription and the synthesis of proteins. These mechanisms of action include: • Potential alteration of the G protein-coupled receptors to interfere with intracellular signal transduction pathways • Enhanced transcription in many genes, especially those involving suppression of inflammation. • Inhibition of gene transcription – including those that encode pro-inflammatory substances. The last two of these are considered genomic effects. This type of corticosteroid effect usually occurs within hours to days after administration. The genomic effects persist after the concentrations of the synthetic corticosteroid in plasma are no longer detectable, as evidenced by persistent suppression of the normal production of hydrocortisone following synthetic corticosteroid administration.6 When used judiciously, corticosteroids can be beneficial to the horse. -

Nitrate Prodrugs Able to Release Nitric Oxide in a Controlled and Selective
Europäisches Patentamt *EP001336602A1* (19) European Patent Office Office européen des brevets (11) EP 1 336 602 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: C07C 205/00, A61K 31/00 20.08.2003 Bulletin 2003/34 (21) Application number: 02425075.5 (22) Date of filing: 13.02.2002 (84) Designated Contracting States: (71) Applicant: Scaramuzzino, Giovanni AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU 20052 Monza (Milano) (IT) MC NL PT SE TR Designated Extension States: (72) Inventor: Scaramuzzino, Giovanni AL LT LV MK RO SI 20052 Monza (Milano) (IT) (54) Nitrate prodrugs able to release nitric oxide in a controlled and selective way and their use for prevention and treatment of inflammatory, ischemic and proliferative diseases (57) New pharmaceutical compounds of general effects and for this reason they are useful for the prep- formula (I): F-(X)q where q is an integer from 1 to 5, pref- aration of medicines for prevention and treatment of in- erably 1; -F is chosen among drugs described in the text, flammatory, ischemic, degenerative and proliferative -X is chosen among 4 groups -M, -T, -V and -Y as de- diseases of musculoskeletal, tegumental, respiratory, scribed in the text. gastrointestinal, genito-urinary and central nervous sys- The compounds of general formula (I) are nitrate tems. prodrugs which can release nitric oxide in vivo in a con- trolled and selective way and without hypotensive side EP 1 336 602 A1 Printed by Jouve, 75001 PARIS (FR) EP 1 336 602 A1 Description [0001] The present invention relates to new nitrate prodrugs which can release nitric oxide in vivo in a controlled and selective way and without the side effects typical of nitrate vasodilators drugs. -

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Record of Reasons for 16-18 November 1999 Meeting
1 NATIONAL DRUGS AND POISONS SCHEDULE COMMITTEE MEETING 25 – 16-18 November 1999 RECORD OF REASONS FOR AMENDMENT TO THE STANDARD FOR THE UNIFORM SCHEDULING OF DRUGS AND POISONS AGRICULTURAL, VETERINARY AND DOMESTIC CHEMICALS Buprofezin – Schedule required Amendment and Reasons The decision below was based on buprofezin’s toxicological profile, in particular its acute oral toxicity. A cut-off to exempt at a concentration of 40% was agreed because of the reduced oral toxicity at this concentration and the ability of safety directions and warning statements applied through the registration system for agricultural and veterinary chemicals to adequately address the slight eye irritation which was attributed to the product formulation. Schedule 5 – New entry BUPROFEZIN except in preparations containing 40 per cent or less of buprofezin. Copper hydroxide - progression of foreshadowed decision to include copper hydroxide in Schedule 6 with cut-off to Schedule 5 at 50 per cent and to exempt at 12.5 per cent. Amendment and Reasons The amendment below was based on the acute oral toxicity of copper hydroxide, its severe eye irritancy / corrosivity, and the possibility of products containing copper hydroxide being accessible in domestic situations and therefore presenting a risk of accidental ingestion. Schedule 6 – New entry COPPER HYDROXIDE except: (a) when included in Schedule 5; or (b) in preparations containing 12.5 per cent or less of copper hydroxide. Schedule 5 – New entry COPPER HYDROXIDE in preparations containing 50 per cent or less of copper hydroxide except in preparations containing 12.5 per cent or less of copper hydroxide. Cyhexatin - Review of Appendix F warning statements and Appendix J rider. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Megestrol Acetate/Melengestrol Acetate 2115 Adverse Effects and Precautions Megestrol Acetate Is Also Used in the Treatment of Ano- 16
Megestrol Acetate/Melengestrol Acetate 2115 Adverse Effects and Precautions Megestrol acetate is also used in the treatment of ano- 16. Mwamburi DM, et al. Comparing megestrol acetate therapy with oxandrolone therapy for HIV-related weight loss: similar As for progestogens in general (see Progesterone, rexia and cachexia (see below) in patients with cancer results in 2 months. Clin Infect Dis 2004; 38: 895–902. p.2125). The weight gain that may occur with meges- or AIDS. The usual dose is 400 to 800 mg daily, as tab- 17. Grunfeld C, et al. Oxandrolone in the treatment of HIV-associ- ated weight loss in men: a randomized, double-blind, placebo- trol acetate appears to be associated with an increased lets or oral suspension. A suspension of megestrol ace- controlled study. J Acquir Immune Defic Syndr 2006; 41: appetite and food intake rather than with fluid reten- tate that has an increased bioavailability is also availa- 304–14. tion. Megestrol acetate may have glucocorticoid ef- ble (Megace ES; Par Pharmaceutical, USA) and is Hot flushes. Megestrol has been used to treat hot flushes in fects when given long term. given in a dose of 625 mg in 5 mL daily for anorexia, women with breast cancer (to avoid the potentially tumour-stim- cachexia, or unexplained significant weight loss in pa- ulating effects of an oestrogen—see Malignant Neoplasms, un- Effects on carbohydrate metabolism. Megestrol therapy der Precautions of HRT, p.2075), as well as in men with hot 1-3 4 tients with AIDS. has been associated with hyperglycaemia or diabetes mellitus flushes after orchidectomy or anti-androgen therapy for prostate in AIDS patients being treated for cachexia. -

A New Robust Technique for Testing of Glucocorticosteroids in Dogs and Horses Terry E
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2007 A new robust technique for testing of glucocorticosteroids in dogs and horses Terry E. Webster Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Veterinary Toxicology and Pharmacology Commons Recommended Citation Webster, Terry E., "A new robust technique for testing of glucocorticosteroids in dogs and horses" (2007). Retrospective Theses and Dissertations. 15029. https://lib.dr.iastate.edu/rtd/15029 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. A new robust technique for testing of glucocorticosteroids in dogs and horses by Terry E. Webster A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Toxicology Program o f Study Committee: Walter G. Hyde, Major Professor Steve Ensley Thomas Isenhart Iowa State University Ames, Iowa 2007 Copyright © Terry Edward Webster, 2007. All rights reserved UMI Number: 1446027 Copyright 2007 by Webster, Terry E. All rights reserved. UMI Microform 1446027 Copyright 2007 by ProQuest Information and Learning Company. All rights reserved. This microform edition is protected against unauthorized copying under Title 17, United States Code. ProQuest Information and Learning Company 300 North Zeeb Road P.O. Box 1346 Ann Arbor, MI 48106-1346 ii DEDICATION I want to dedicate this project to my wife, Jackie, and my children, Shauna, Luke and Jake for their patience and understanding without which this project would not have been possible. -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

The Inhaled Steroid Ciclesonide Blocks SARS-Cov-2 RNA Replication by Targeting Viral
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting viral 2 replication-transcription complex in culture cells 3 4 Shutoku Matsuyamaa#, Miyuki Kawasea, Naganori Naoa, Kazuya Shiratoa, Makoto Ujikeb, Wataru 5 Kamitanic, Masayuki Shimojimad, and Shuetsu Fukushid 6 7 aDepartment of Virology III, National Institute of Infectious Diseases, Tokyo, Japan 8 bFaculty of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan 9 cDepartment of Infectious Diseases and Host Defense, Gunma University Graduate School of 10 Medicine, Gunma, Japan 11 dDepartment of Virology I, National Institute of Infectious Diseases, Tokyo, Japan. 12 13 Running Head: Ciclesonide blocks SARS-CoV-2 replication 14 15 #Address correspondence to Shutoku Matsuyama, [email protected] 16 17 Word count: Abstract 149, Text 3,016 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 18 Abstract 19 We screened steroid compounds to obtain a drug expected to block host inflammatory responses and 20 MERS-CoV replication. Ciclesonide, an inhaled corticosteroid, suppressed replication of MERS-CoV 21 and other coronaviruses, including SARS-CoV-2, the cause of COVID-19, in cultured cells. The 22 effective concentration (EC90) of ciclesonide for SARS-CoV-2 in differentiated human bronchial 23 tracheal epithelial cells was 0.55 μM. -

Effective July 1, 2011
MISSISSIPPI DIVISION OF MEDICAID PREFERRED DRUG LIST Effective July 1, 2011 BODY SYSTEM THERAPEUTIC CLASS PREFERRED AGENTS NON-PREFERRED AGENTS NOTES ANALGESICS ANALGESICS, fentanyl patches AVINZA (morphine) NARCOTIC-LONG-ACTING KADIAN (morphine) BUTRANS (buprenorphine) methadone DURAGESIC (fentanyl) morphine ER EMBEDA (morphine/naltrexone) EXALGO (hydromorphone) OPANA ER (oxymorphone) oxycodone ER OXYCONTIN (oxycodone) RYZOLT (tramadol) ULTRAM ER (tramadol) ANALGESICS, NARCOTIC- acetaminophen/codeine ABSTRAL (fentanyl) SHORT-ACTING aspirin/codeine butalbital/APAP/caffeine/codeine codeine butalbital/ASA/caffeine/codeine dihydrocodeine/ APAP/caffeine DARVON-N (propoxyphene) hydrocodone/APAP DILAUDID liquid (hydromorphone) hydrocodone/ibuprofen fentanyl hydromorphone FENTORA (fentanyl) IBUDONE (hydrocodone/ibuprofen) levorphanol meperidine NUCYNTA (tapentadol) morphine ONSOLIS (fentanyl) oxycodone OPANA (oxymorphone) oxycodone/APAP pentazocine/naloxone oxycodone/aspirin propoxyphene oxycodone/ibuprofen propoxyphene/APAP pentazocine/APAP REPREXAIN tramadol (hydrocodone/ibuprofen) tramadol/APAP RYBIX (tramadol) VIMOVO (naproxen/esomeprazole) ZAMICET (hydrocodone/APAP) ZOLVIT (hydrocodone/APAP) ANALGESICS/ANESTHETICS, FLECTOR (diclofenac epolamine) PENNSAID Solution TOPICAL LIDODERM (lidocaine) (diclofenac sodium ) VOLTAREN Gel (diclofenac sodium) ANTIHYPERURICEMICS allopurinol ULORIC (febuxostat) COLCRYS (colchicine) probenecid probenecid/colchicine ANALGESICS (continued) Unless otherwise stated, the listing of a particular brand or generic -

University of Groningen Multi-Residue Analysis of Growth Promotors In
University of Groningen Multi-residue analysis of growth promotors in food-producing animals Koole, Anneke IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 1998 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Koole, A. (1998). Multi-residue analysis of growth promotors in food-producing animals. s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 25-09-2021 APPENDIX 1 OVERVIEW OF RELEVANT SUBSTANCES This appendix consists of two parts. First, substances that are relevant for the research presented in this thesis are given. For each substance CAS number (CAS), molecular weight (MW), bruto formula (formula) and if available UV maxima and alternative names are given. In addition, pKa values for the ß-agonists are listed, if they were available. -
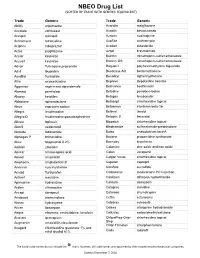
Drug List (SORTED by TRADE with GENERIC EQUIVALENT)
NBEO Drug List (SORTED BY TRADE WITH GENERIC EQUIVALENT) Trade Generic Trade Generic Abilify aripiprazole Avandia rosiglitazone Accolate zafirlukast Avastin bevacizumab Accupril quinapril Azasan azathioprine Achromycin tetracycline AzaSite azithromycin Aciphex rabeprazole Avodart dutasteride Actos pioglitazone Azopt brinzolamide Acular ketorolac Bactrim trimethoprim-sulfamethoxazole Acuvail ketorolac Bactrim DS trimethoprim-sulfamethoxazole Advair fluticasone propionate Baquacil polyhexamethylene biguanide Advil ibuprofen Beconase AQ beclomethasone AeroBid flunisolide Benadryl diphenhydramine Afrin oxymetazoline Bepreve Bepotastine besilate Aggrenox aspirin and dipyridamole Besivance besifloxacin Alamast pemirolast Betadine povidone-iodine Alaway ketotifen Betagan levobunolol Aldactone spironolactone Betasept chlorhexidine topical Aleve naproxen sodium Betaseron interferon beta-1b Allegra fexofenadine Betimol timolol Allegra-D fexofenadine-pseudoephedrine Betoptic S betaxolol Alluvia lopinavir Biopatch chlorhexidine topical Alocril nedocromil Blephamide sulfacetamide-prednisolone Alomide lodoxamide Botox onabotulinum toxinA Alphagan P brimonidine Brolene propamidine isethionate Alrex loteprednol 0.2% Bromday bromfenac Ambien zolpidem Calamine zinc oxide and iron oxide Amicar aminocaproic acid Calan verapamil Amoxil amoxicillin Calgon Vesta chlorhexidine topical Amphocin amphotericin B Capoten captopril Anectine succinylcholine Carafate sucralfate Ansaid flurbiprofen Carbocaine mepivacaine HCl injection Antivert meclizine Cardizem diltiazem