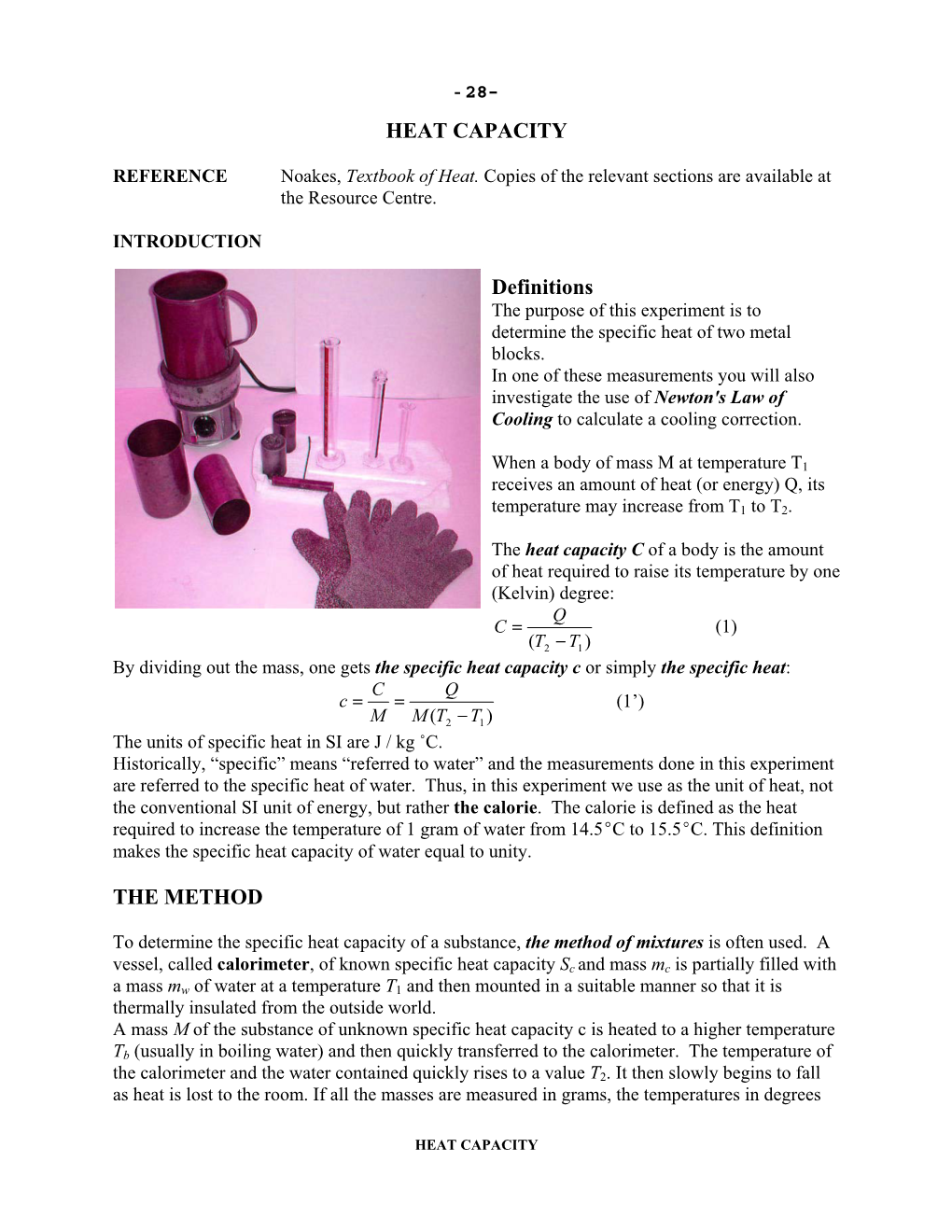
HEAT CAPACITY Definitions the METHOD
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Soda Can Calorimeter
Soda Can Calorimeter Energy Content of Food SCIENTIFIC SCIENCEFAX! Introduction Have you ever noticed the nutrition label located on the packaging of the food you buy? One of the first things listed on the label are the calories per serving. How is the calorie content of food determined? This activity will introduce the concept of calorimetry and investigate the caloric content of snack foods. Concepts •Calorimetry • Conservation of energy • First law of thermodynamics Background The law of conservation of energy states that energy cannot be created or destroyed, only converted from one form to another. This fundamental law was used by scientists to derive new laws in the field of thermodynamics—the study of heat energy, temperature, and heat transfer. The First Law of Thermodynamics states that the heat energy lost by one body is gained by another body. Heat is the energy that is transferred between objects when there is a difference in temperature. Objects contain heat as a result of the small, rapid motion (vibrations, rotational motion, electron spin, etc.) that all atoms experience. The temperature of an object is an indirect measurement of its heat. Particles in a hot object exhibit more rapid motion than particles in a colder object. When a hot and cold object are placed in contact with one another, the faster moving particles in the hot object will begin to bump into the slower moving particles in the colder object making them move faster (vice versa, the faster particles will then move slower). Eventually, the two objects will reach the same equilibrium temperature—the initially cold object will now be warmer, and the initially hot object will now be cooler. -

Lab: Specific Heat
Lab: Specific Heat Summary Students relate thermal energy to heat capacity by comparing the heat capacities of different materials and graphing the change in temperature over time for a specific material. Students explore the idea of insulators and conductors in an attempt to apply their knowledge in real world applications, such as those that engineers would. Engineering Connection Engineers must understand the heat capacity of insulators and conductors if they wish to stay competitive in “green” enterprises. Engineers who design passive solar heating systems take advantage of the natural heat capacity characteristics of materials. In areas of cooler climates engineers chose building materials with a low heat capacity such as slate roofs in an attempt to heat the home during the day. In areas of warmer climates tile roofs are popular for their ability to store the sun's heat energy for an extended time and release it slowly after the sun sets, to prevent rapid temperature fluctuations. Engineers also consider heat capacity and thermal energy when they design food containers and household appliances. Just think about your aluminum soda can versus glass bottles! Grade Level: 9-12 Group Size: 4 Time Required: 90 minutes Activity Dependency :None Expendable Cost Per Group : US$ 5 The cost is less if thermometers are available for each group. NYS Science Standards: STANDARD 1 Analysis, Inquiry, and Design STANDARD 4 Performance indicator 2.2 Keywords: conductor, energy, energy storage, heat, specific heat capacity, insulator, thermal energy, thermometer, thermocouple Learning Objectives After this activity, students should be able to: Define heat capacity. Explain how heat capacity determines a material's ability to store thermal energy. -

What Is a Calorie?
What Is A Calorie? Guide Copyright © 2008 Learning Seed Suite 301 641 West Lake Street Chicago, IL 60661 800.634.4941 [email protected] www.learningseed.com What Is A Calorie? Legal Niceties The Video Copyright © 2005 Learning Seed. This video program is protected under U.S. copyright law. No part of this video may be reproduced or transmitted by any means, electronic or mechanical, without the written permission of the Publisher, except where permitted by law. This Teaching Guide Copyright © 2008 Learning Seed. This teaching guide is copyrighted according to the terms of the Creative Commons non-commercial license (http://creativecommons.org/licenses/by-nc/2.5/). It may be reproduced, in its part or its entirety, for classroom use. No part of this guide may be reproduced for sale by any party. You are free: • to copy, distribute, display, and perform the work. • to make derivative works. Under the following conditions: • Attribution. You must attribute the work to Learning Seed. • Noncommercial. You may not use this work for commercial purposes. • For any reuse or distribution, you must make clear to others the license terms of this work. • Any of these conditions can be waived if you get permission from the copyright holder. Credits This Teaching Guide Compilation: Rebecca Phipps Copy Editor: Jennifer Smith Learning Seed Catalog and ISBN Numbers Please contact us with any questions or concerns at: VHS LS-1284-05-VHS ISBN 0-917159-75-6 Learning Seed DVD LS-1284-05-DVD ISBN 0-917159-23-3 Suite 301 641 West Lake Street Chicago, IL 60661 800.634.4941 [email protected] Closed Captioning This program is closed-captioned. -

Lecture 4: 09.16.05 Temperature, Heat, and Entropy
3.012 Fundamentals of Materials Science Fall 2005 Lecture 4: 09.16.05 Temperature, heat, and entropy Today: LAST TIME .........................................................................................................................................................................................2� State functions ..............................................................................................................................................................................2� Path dependent variables: heat and work..................................................................................................................................2� DEFINING TEMPERATURE ...................................................................................................................................................................4� The zeroth law of thermodynamics .............................................................................................................................................4� The absolute temperature scale ..................................................................................................................................................5� CONSEQUENCES OF THE RELATION BETWEEN TEMPERATURE, HEAT, AND ENTROPY: HEAT CAPACITY .......................................6� The difference between heat and temperature ...........................................................................................................................6� Defining heat capacity.................................................................................................................................................................6� -

Compression of an Ideal Gas with Temperature Dependent Specific
Session 3666 Compression of an Ideal Gas with Temperature-Dependent Specific Heat Capacities Donald W. Mueller, Jr., Hosni I. Abu-Mulaweh Engineering Department Indiana University–Purdue University Fort Wayne Fort Wayne, IN 46805-1499, USA Overview The compression of gas in a steady-state, steady-flow (SSSF) compressor is an important topic that is addressed in virtually all engineering thermodynamics courses. A typical situation encountered is when the gas inlet conditions, temperature and pressure, are known and the compressor dis- charge pressure is specified. The traditional instructional approach is to make certain assumptions about the compression process and about the gas itself. For example, a special case often consid- ered is that the compression process is reversible and adiabatic, i.e. isentropic. The gas is usually ÊÌ considered to be ideal, i.e. ÈÚ applies, with either constant or temperature-dependent spe- cific heat capacities. The constant specific heat capacity assumption allows for direct computation of the discharge temperature, while the temperature-dependent specific heat assumption does not. In this paper, a MATLAB computer model of the compression process is presented. The substance being compressed is air which is considered to be an ideal gas with temperature-dependent specific heat capacities. Two approaches are presented to describe the temperature-dependent properties of air. The first approach is to use the ideal gas table data from Ref. [1] with a look-up interpolation scheme. The second approach is to use a curve fit with the NASA Lewis coefficients [2,3]. The computer model developed makes use of a bracketing–bisection algorithm [4] to determine the temperature of the air after compression. -

Energy Equilibrium Energy Balance Calorie Referstoa the Riseintemperature Withathermometer
Energy Balance Energy Equilibrium Did you know that the food we eat provides us with energy? We use energy 24 hours a day, seven days a which means the food really contains 110,000 week. Each person needs a different amount of calories. However, food labels in the United energy, and that amount changes throughout States use Calories (capitalized), which is the our lives. The idea is to consume just enough same as kilocalories. Internationally, the energy energy for our bodies to function; not too much measurement of kilojoules is used. It is another or too little. We need to find a balance between way to measure the same item. One kilojoule is energy burned and energy consumed because approximately equal to 0.25 kilocalories. excess energy is stored in our bodies as fat. To find out exactly how many calories are in The energy we consume is measured in food, scientists can use a bomb calorimeter. A kilocalories (kcal). A kilocalorie is defined as bomb calorimeter measures the heat released the amount of energy required to raise the when food is burned, which provides an estimate temperature of a liter of water one degree of potential energy in the food. The amount Celsius. You may have heard of calories instead of energy an object possibly has is known as of kilocalories. The term calorie refers to a potential energy. The bomb calorimeter is used unit of food energy. This energy is expressed because it mimics the method our body uses to in 1000-calorie metric units, known as a break down food. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

What Are My Calorie, Protein, Fat, & Carbohydrate Needs
What are My Calorie, Protein, Fat, & Carbohydrate Needs? Calorie Needs The Harris-Benedict Equation for Basal Energy Expenditure (BEE) is commonly used to figure energy requirements based on sex, height, weight and age. W = weight in kilograms H = height in centimeters A = age in years Men: BEE = 66.5 + 13.8(W) + 5.0(H) - 6.8(A) Women: BEE = 655.1 + 9.6(W) + 1.9(H) - 4.7(A) Example: Beth is a 47 year-old female. She is 5’5” tall and weighs 147 pounds. What is her BEE? (147 pounds)/(2.2 pounds/kg) = 66.8 kg (65 inches)(2.54 cm/inch) = 165 cm BEE = 655.1 + [(9.6)(66.8 kg)] + [(5.0)(165 cm)] - [(4.7)(47 age)] BEE = 655.1 + 641 + 825 – 221 = 1900 calories If Beth gets regular physical activity, her BEE may need to be multiplied by a factor of 1.2-1.5 to account for extra calories needed during exercise. A factor of 1.2 represents an average amount of activity, where 1.5 would be a very high amount of activity. We’ll say Beth gets an average amount of activity. In this case her needs would be: 1900 calories (1.2) = 2280 calories Protein Needs The American Dietetic Association (ADA) recommends daily protein intake for healthy adults as .8-1.0 g of protein/kg body weight. Example: Jay weighs 168 pounds. How much protein does he need per day? (168)/(2.2g/kg) = 76.4 kg 76.4 kg (.8 g/kg) = 61 g 76.4 kg (1.0 g/kg) = 76 g 61 – 76 grams of protein per day Fat Needs Fat intake should equal 30% of your total days calories. -

Entropy Determination of Single-Phase High Entropy Alloys with Different Crystal Structures Over a Wide Temperature Range
entropy Article Entropy Determination of Single-Phase High Entropy Alloys with Different Crystal Structures over a Wide Temperature Range Sebastian Haas 1, Mike Mosbacher 1, Oleg N. Senkov 2 ID , Michael Feuerbacher 3, Jens Freudenberger 4,5 ID , Senol Gezgin 6, Rainer Völkl 1 and Uwe Glatzel 1,* ID 1 Metals and Alloys, University Bayreuth, 95447 Bayreuth, Germany; [email protected] (S.H.); [email protected] (M.M.); [email protected] (R.V.) 2 UES, Inc., 4401 Dayton-Xenia Rd., Dayton, OH 45432, USA; [email protected] 3 Institut für Mikrostrukturforschung, Forschungszentrum Jülich, 52425 Jülich, Germany; [email protected] 4 Leibniz Institute for Solid State and Materials Research Dresden (IFW Dresden), 01069 Dresden, Germany; [email protected] 5 TU Bergakademie Freiberg, Institut für Werkstoffwissenschaft, 09599 Freiberg, Germany 6 NETZSCH Group, Analyzing & Testing, 95100 Selb, Germany; [email protected] * Correspondence: [email protected]; Tel.: +49-921-55-5555 Received: 31 July 2018; Accepted: 20 August 2018; Published: 30 August 2018 Abstract: We determined the entropy of high entropy alloys by investigating single-crystalline nickel and five high entropy alloys: two fcc-alloys, two bcc-alloys and one hcp-alloy. Since the configurational entropy of these single-phase alloys differs from alloys using a base element, it is important to quantify ◦ the entropy. Using differential scanning calorimetry, cp-measurements are carried out from −170 C to the materials’ solidus temperatures TS. From these experiments, we determined the thermal entropy and compared it to the configurational entropy for each of the studied alloys. -

THERMOCHEMISTRY – 2 CALORIMETRY and HEATS of REACTION Dr
THERMOCHEMISTRY – 2 CALORIMETRY AND HEATS OF REACTION Dr. Sapna Gupta HEAT CAPACITY • Heat capacity is the amount of heat needed to raise the temperature of the sample of substance by one degree Celsius or Kelvin. q = CDt • Molar heat capacity: heat capacity of one mole of substance. • Specific Heat Capacity: Quantity of heat needed to raise the temperature of one gram of substance by one degree Celsius (or one Kelvin) at constant pressure. q = m s Dt (final-initial) • Measured using a calorimeter – it absorbed heat evolved or absorbed. Dr. Sapna Gupta/Thermochemistry-2-Calorimetry 2 EXAMPLES OF SP. HEAT CAPACITY The higher the number the higher the energy required to raise the temp. Dr. Sapna Gupta/Thermochemistry-2-Calorimetry 3 CALORIMETRY: EXAMPLE - 1 Example: A piece of zinc weighing 35.8 g was heated from 20.00°C to 28.00°C. How much heat was required? The specific heat of zinc is 0.388 J/(g°C). Solution m = 35.8 g s = 0.388 J/(g°C) Dt = 28.00°C – 20.00°C = 8.00°C q = m s Dt 0.388 J q 35.8 g 8.00C = 111J gC Dr. Sapna Gupta/Thermochemistry-2-Calorimetry 4 CALORIMETRY: EXAMPLE - 2 Example: Nitromethane, CH3NO2, an organic solvent burns in oxygen according to the following reaction: 3 3 1 CH3NO2(g) + /4O2(g) CO2(g) + /2H2O(l) + /2N2(g) You place 1.724 g of nitromethane in a calorimeter with oxygen and ignite it. The temperature of the calorimeter increases from 22.23°C to 28.81°C. -

Module P7.4 Specific Heat, Latent Heat and Entropy
FLEXIBLE LEARNING APPROACH TO PHYSICS Module P7.4 Specific heat, latent heat and entropy 1 Opening items 4 PVT-surfaces and changes of phase 1.1 Module introduction 4.1 The critical point 1.2 Fast track questions 4.2 The triple point 1.3 Ready to study? 4.3 The Clausius–Clapeyron equation 2 Heating solids and liquids 5 Entropy and the second law of thermodynamics 2.1 Heat, work and internal energy 5.1 The second law of thermodynamics 2.2 Changes of temperature: specific heat 5.2 Entropy: a function of state 2.3 Changes of phase: latent heat 5.3 The principle of entropy increase 2.4 Measuring specific heats and latent heats 5.4 The irreversibility of nature 3 Heating gases 6 Closing items 3.1 Ideal gases 6.1 Module summary 3.2 Principal specific heats: monatomic ideal gases 6.2 Achievements 3.3 Principal specific heats: other gases 6.3 Exit test 3.4 Isothermal and adiabatic processes Exit module FLAP P7.4 Specific heat, latent heat and entropy COPYRIGHT © 1998 THE OPEN UNIVERSITY S570 V1.1 1 Opening items 1.1 Module introduction What happens when a substance is heated? Its temperature may rise; it may melt or evaporate; it may expand and do work1—1the net effect of the heating depends on the conditions under which the heating takes place. In this module we discuss the heating of solids, liquids and gases under a variety of conditions. We also look more generally at the problem of converting heat into useful work, and the related issue of the irreversibility of many natural processes. -

1. Energy and Power1
1. Energy and Power1 © John Dawson At the end of the Cretaceous period, the golden age of dinosaurs, an asteroid or comet about 10 miles in diameter headed directly towards the Earth with a velocity of about 20 miles per second, over ten times faster than our speediest bullets. Many such large objects may have come close to the Earth, but this was the one that finally hit. It hardly noticed the air as it plunged through the atmosphere in a fraction of a second, momentarily leaving a trail of vacuum behind it. It hit the Earth with such force that it and the rock near it were suddenly heated to a temperature of over a million degrees Centigrade, several hundred times hotter than the surface of the sun. Asteroid, rock, and water (if it hit in the ocean) were instantly vaporized. The energy released in the explosion was greater than that of a hundred million megatons of TNT, 100 teratons, more than ten thousand times greater than the total U.S. and Soviet nuclear arsenals. Before a minute had passed, the expanding crater was 60 miles across and 20 miles deep. It would soon grow even larger. Hot vaporized material from the impact had already blasted its way out through most of the atmosphere to an altitude of 15 miles. Material that a moment earlier had been glowing plasma was beginning to cool and condense into dust and rock that would be spread worldwide. Few people are surprised by the fact that an asteroid, the size of Mt. Everest, could do a lot of damage when it hits the Earth.