Dogwood Anthracnose and Its Spread in the South
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Diseases of Trees in the Great Plains
United States Department of Agriculture Diseases of Trees in the Great Plains Forest Rocky Mountain General Technical Service Research Station Report RMRS-GTR-335 November 2016 Bergdahl, Aaron D.; Hill, Alison, tech. coords. 2016. Diseases of trees in the Great Plains. Gen. Tech. Rep. RMRS-GTR-335. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 229 p. Abstract Hosts, distribution, symptoms and signs, disease cycle, and management strategies are described for 84 hardwood and 32 conifer diseases in 56 chapters. Color illustrations are provided to aid in accurate diagnosis. A glossary of technical terms and indexes to hosts and pathogens also are included. Keywords: Tree diseases, forest pathology, Great Plains, forest and tree health, windbreaks. Cover photos by: James A. Walla (top left), Laurie J. Stepanek (top right), David Leatherman (middle left), Aaron D. Bergdahl (middle right), James T. Blodgett (bottom left) and Laurie J. Stepanek (bottom right). To learn more about RMRS publications or search our online titles: www.fs.fed.us/rm/publications www.treesearch.fs.fed.us/ Background This technical report provides a guide to assist arborists, landowners, woody plant pest management specialists, foresters, and plant pathologists in the diagnosis and control of tree diseases encountered in the Great Plains. It contains 56 chapters on tree diseases prepared by 27 authors, and emphasizes disease situations as observed in the 10 states of the Great Plains: Colorado, Kansas, Montana, Nebraska, New Mexico, North Dakota, Oklahoma, South Dakota, Texas, and Wyoming. The need for an updated tree disease guide for the Great Plains has been recog- nized for some time and an account of the history of this publication is provided here. -

Dogwood Anthracnose by the Bartlett Lab Staff
RESEARCH LABORATORY TECHNICAL REPORT Dogwood Anthracnose By The Bartlett Lab Staff Anthracnose caused by the fungus Discula destructiva is a potentially fatal disease of dogwood. All varieties of the native flowering dogwood (Cornus florida and C. nuttallii) are susceptible. The disease usually starts on lower leaves and progresses into twigs and branches. Infected trees are severely weakened so that secondary canker and root rot diseases can infect and kill the tree. Discula infection usually occurs during cool, rainy periods in the spring. The fungal spores are spread by rain and wind. Symptoms branches (water sprouts) are more susceptible to the fungus. Often, the first symptoms of Discula destructiva anthracnose are spots on the lower leaves and flower The speed at which the disease progresses in the tree bracts. Spots are tan to brown and may have purple depends on weather, tree health, and treatment. With rings around them (Figure 1). This symptom is similar weather favorable to the disease and no treatments, to small spots caused by the fungus Elsinoe corni. If the most infected trees are killed within 3 to 6 years. infection is caused by Discula, leaf tissue will also be killed along the veins or the entire leaf will be killed. Management Those leaves which succumb to infection during the Healthy dogwoods are able to withstand disease summer will stay on the plant after normal leaf. The infection much better than stressed trees. To keep trees infection will also spread from the leaves to the twigs vigorous they should be mulched, watered, fertilized, resulting in cankers and twig dieback. -

Fungi and Their Potential As Biological Control Agents of Beech Bark Disease
Fungi and their potential as biological control agents of Beech Bark Disease By Sarah Elizabeth Thomas A thesis submitted for the degree of Doctor of Philosophy School of Biological Sciences Royal Holloway, University of London 2014 1 DECLARATION OF AUTHORSHIP I, Sarah Elizabeth Thomas, hereby declare that this thesis and the work presented in it is entirely my own. Where I have consulted the work of others, this is always clearly stated. Signed: _____________ Date: 4th May 2014 2 ABSTRACT Beech bark disease (BBD) is an invasive insect and pathogen disease complex that is currently devastating American beech (Fagus grandifolia) in North America. The disease complex consists of the sap-sucking scale insect, Cryptococcus fagisuga and sequential attack by Neonectria fungi (principally Neonectria faginata). The scale insect is not native to North America and is thought to have been introduced there on seedlings of F. sylvatica from Europe. Conventional control strategies are of limited efficacy in forestry systems and removal of heavily infested trees is the only successful method to reduce the spread of the disease. However, an alternative strategy could be the use of biological control, using fungi. Fungal endophytes and/or entomopathogenic fungi (EPF) could have potential for both the insect and fungal components of this highly invasive disease. Over 600 endophytes were isolated from healthy stems of F. sylvatica and 13 EPF were isolated from C. fagisuga cadavers in its centre of origin. A selection of these isolates was screened in vitro for their suitability as biological control agents. Two Beauveria and two Lecanicillium isolates were assessed for their suitability as biological control agents for C. -
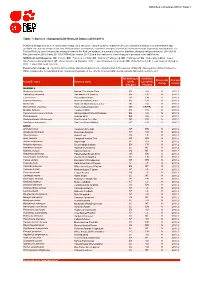
Table 7: Species Changing IUCN Red List Status (2010-2011)
IUCN Red List version 2011.2: Table 7 Table 7: Species changing IUCN Red List Status (2010-2011) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2010 (IUCN Red List version 2010.4) and 2011 (IUCN Red List version 2011.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2010) List (2011) change version Category Category MAMMALS Bradypus torquatus Maned Three-toed Sloth EN VU N 2011.1 Callicebus oenanthe San Martin Titi Monkey EN CR N 2011.1 Equus ferus Przewalski's Horse CR EN G 2011.2 -

<I>Discula Destructiva</I>
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 5-2011 Infection Process of Discula destructiva, the Causal Agent of Dogwood Anthracnose, and Resistance Mechanism of Flowering Dogwood Qunkang Cheng [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Plant Pathology Commons Recommended Citation Cheng, Qunkang, "Infection Process of Discula destructiva, the Causal Agent of Dogwood Anthracnose, and Resistance Mechanism of Flowering Dogwood. " Master's Thesis, University of Tennessee, 2011. https://trace.tennessee.edu/utk_gradthes/864 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Qunkang Cheng entitled "Infection Process of Discula destructiva, the Causal Agent of Dogwood Anthracnose, and Resistance Mechanism of Flowering Dogwood." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Entomology and Plant Pathology. Mark T. Windham, Major Professor We have read this thesis and recommend its acceptance: Alan S. Windham, William E. Klingeman, Arnold M. Saxton Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Infection Process of Discula destructiva, the Causal Agent of Dogwood Anthracnose, and Resistance Mechanism of Flowering Dogwood A Thesis Presented for the Master of Science Degree The University of Tennessee, Knoxville Qunkang Cheng May 2011 ACKNOWLEDGEMENTS I wish to express my sincerest appreciation to my major professor, Dr. -

European Red List of Non-Marine Molluscs Annabelle Cuttelod, Mary Seddon and Eike Neubert
European Red List of Non-marine Molluscs Annabelle Cuttelod, Mary Seddon and Eike Neubert European Red List of Non-marine Molluscs Annabelle Cuttelod, Mary Seddon and Eike Neubert IUCN Global Species Programme IUCN Regional Office for Europe IUCN Species Survival Commission Published by the European Commission. This publication has been prepared by IUCN (International Union for Conservation of Nature) and the Natural History of Bern, Switzerland. The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN, the Natural History Museum of Bern or the European Union concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN, the Natural History Museum of Bern or the European Commission. Citation: Cuttelod, A., Seddon, M. and Neubert, E. 2011. European Red List of Non-marine Molluscs. Luxembourg: Publications Office of the European Union. Design & Layout by: Tasamim Design - www.tasamim.net Printed by: The Colchester Print Group, United Kingdom Picture credits on cover page: The rare “Hélice catalorzu” Tacheocampylaea acropachia acropachia is endemic to the southern half of Corsica and is considered as Endangered. Its populations are very scattered and poor in individuals. This picture was taken in the Forêt de Muracciole in Central Corsica, an occurrence which was known since the end of the 19th century, but was completely destroyed by a heavy man-made forest fire in 2000. -

Discula Lyelliana
The IUCN Red List of Threatened Species™ ISSN 2307-8235 (online) IUCN 2008: T6730A12801850 Discula lyelliana Assessment by: Seddon, M. View on www.iucnredlist.org Citation: Seddon, M. 2013. Discula lyelliana. The IUCN Red List of Threatened Species 2013: e.T6730A12801850. http://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T6730A12801850.en Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale, reposting or other commercial purposes is prohibited without prior written permission from the copyright holder. For further details see Terms of Use. The IUCN Red List of Threatened Species™ is produced and managed by the IUCN Global Species Programme, the IUCN Species Survival Commission (SSC) and The IUCN Red List Partnership. The IUCN Red List Partners are: BirdLife International; Botanic Gardens Conservation International; Conservation International; Microsoft; NatureServe; Royal Botanic Gardens, Kew; Sapienza University of Rome; Texas A&M University; Wildscreen; and Zoological Society of London. If you see any errors or have any questions or suggestions on what is shown in this document, please provide us with feedback so that we can correct or extend the information provided. THE IUCN RED LIST OF THREATENED SPECIES™ Taxonomy Kingdom Phylum Class Order Family Animalia Mollusca Gastropoda Stylommatophora Hygromiidae Taxon Name: Discula lyelliana (R.T. Lowe, 1852) Assessment Information Red List Category & Criteria: Critically Endangered (Possibly Extinct) B1ab(iii) ver 3.1 Year Published: 2013 Date Assessed: August 31, 2010 Justification: Seddon (2008) suggested this species should be considered as Critically Endangered B1ab(iii) (version 3.1). -

Porto Santo Candidatura a Reserva Da Biosfera Da UNESCO Março De
Porto Santo Candidatura a Reserva da Biosfera da UNESCO Março de 2019 FICHA TÉCNICA COORDENAÇÃO GERAL Município do Porto Santo Associação Grupo de Folclore do Porto Santo Agência Regional da Energia e Ambiente da Região Autónoma da Madeira Direção Regional para a Administração Pública do Porto Santo Instituto das Florestas e Conservação da Natureza, IP-RAM Secretaria Regional do Ambiente e Recursos Naturais EQUIPA TÉCNICA Duarte Mendonça Filipe Oliveira José Manuel Silva Maria Gorete Freitas Rosa Pires Rubina Brito Rute Areal Susana Fontinha DESIGN E PAGINAÇÃO Neide Paixão Núria Brito FOTOS Dos promotores com exceção das identificadas por: DT – Dinarte Teixeira 4 FV – Filipe Viveiros NP – Neide Paixão NS – Nuno Sá RSM – Rui São Marcos VG – Virgílio Gomes COLABORADORES Ana Gomes Ana Luisa Fernandes António Albuquerque e Silva António Franquinho Aguiar António Iglésias Bruno Cunha Daniel Mata Dília Menezes Dinarte Teixeira Eunice Pinto Francisco Clode Francisco Fernandes Gina Brito Mendes João Batista João Delgado João Rodrigues José Augusto Carvalho José Luís Ferreira Lídia Góes Ferreira 5 Manuel Ara Manuela Sim-Sim Mário Cachão Miguel Ângelo Carvalho Olinda Simone Vasconcelos Raquel Ferreira Ricardo Costa Ricardo Meneses Rui Nunes Sara Andrade Vitor Jorge Vítor Prior AGRADECIMENTOS Direção Regional de Agricultura Direcção Regional da Cultura Direção Regional de Estatística da Madeira Direção Regional de Pescas Direção Regional do Turismo Museu Etnográfico da Madeira Um agradecimento especial aos Porto-santenses que se envolveram neste -

Savoryellales (Hypocreomycetidae, Sordariomycetes): a Novel Lineage
Mycologia, 103(6), 2011, pp. 1351–1371. DOI: 10.3852/11-102 # 2011 by The Mycological Society of America, Lawrence, KS 66044-8897 Savoryellales (Hypocreomycetidae, Sordariomycetes): a novel lineage of aquatic ascomycetes inferred from multiple-gene phylogenies of the genera Ascotaiwania, Ascothailandia, and Savoryella Nattawut Boonyuen1 Canalisporium) formed a new lineage that has Mycology Laboratory (BMYC), Bioresources Technology invaded both marine and freshwater habitats, indi- Unit (BTU), National Center for Genetic Engineering cating that these genera share a common ancestor and Biotechnology (BIOTEC), 113 Thailand Science and are closely related. Because they show no clear Park, Phaholyothin Road, Khlong 1, Khlong Luang, Pathumthani 12120, Thailand, and Department of relationship with any named order we erect a new Plant Pathology, Faculty of Agriculture, Kasetsart order Savoryellales in the subclass Hypocreomyceti- University, 50 Phaholyothin Road, Chatuchak, dae, Sordariomycetes. The genera Savoryella and Bangkok 10900, Thailand Ascothailandia are monophyletic, while the position Charuwan Chuaseeharonnachai of Ascotaiwania is unresolved. All three genera are Satinee Suetrong phylogenetically related and form a distinct clade Veera Sri-indrasutdhi similar to the unclassified group of marine ascomy- Somsak Sivichai cetes comprising the genera Swampomyces, Torpedos- E.B. Gareth Jones pora and Juncigera (TBM clade: Torpedospora/Bertia/ Mycology Laboratory (BMYC), Bioresources Technology Melanospora) in the Hypocreomycetidae incertae -

Discula Tectiformis
The IUCN Red List of Threatened Species™ ISSN 2307-8235 (online) IUCN 2008: T6729A12801643 Discula tectiformis Assessment by: Seddon, M.B. View on www.iucnredlist.org Citation: Seddon, M.B. 2013. Discula tectiformis. The IUCN Red List of Threatened Species 2013: e.T6729A12801643. http://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T6729A12801643.en Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale, reposting or other commercial purposes is prohibited without prior written permission from the copyright holder. For further details see Terms of Use. The IUCN Red List of Threatened Species™ is produced and managed by the IUCN Global Species Programme, the IUCN Species Survival Commission (SSC) and The IUCN Red List Partnership. The IUCN Red List Partners are: BirdLife International; Botanic Gardens Conservation International; Conservation International; Microsoft; NatureServe; Royal Botanic Gardens, Kew; Sapienza University of Rome; Texas A&M University; Wildscreen; and Zoological Society of London. If you see any errors or have any questions or suggestions on what is shown in this document, please provide us with feedback so that we can correct or extend the information provided. THE IUCN RED LIST OF THREATENED SPECIES™ Taxonomy Kingdom Phylum Class Order Family Animalia Mollusca Gastropoda Stylommatophora Hygromiidae Taxon Name: Discula tectiformis (Sowerby, 1824) Assessment Information Red List Category & Criteria: Endangered B1ab(iii) ver 3.1 Year Published: 2013 Date Assessed: September 30, 2010 Justification: This species has a total extent of occurrence of 42 km2 with the four isolated sub-populations, three on a small hillslope area at the eastern part of the island. -

Impacts of an Exotic Disease and Vegetation Change on Foliar Calcium Cycling in Appalachian Forests
Ecological Applications, 17(3), 2007, pp. 869–881 Ó 2007 by the Ecological Society of America IMPACTS OF AN EXOTIC DISEASE AND VEGETATION CHANGE ON FOLIAR CALCIUM CYCLING IN APPALACHIAN FORESTS 1,4 2 3 MICHAEL A. JENKINS, SHIBU JOSE, AND PETER S. WHITE 1National Park Service, Great Smoky Mountains National Park, Twin Creeks Natural Resources Center, 1314 Cherokee Orchard Road, Gatlinburg, Tennessee 37738 USA 2School of Forest Resources and Conservation, University of Florida, Box 110410, Gainesville, Florida 32611 USA 3Department of Biology–Campus Box 3280, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599-3280 USA Abstract. Because of the high calcium content of its foliage, Cornus florida (flowering dogwood) has been described as a calcium ‘‘pump’’ that draws calcium from deeper mineral soil and enriches surface soil horizons. However, over the last two decades an exotic fungal disease (dogwood anthracnose, Discula destructiva) has decimated populations of this once- common understory species. Its loss, combined with forest stand development, could alter intra-stand calcium cycling. We used data from long-term vegetation monitoring plots to examine the ecological role of C. florida in calcium cycling and to identify changes in annual foliar calcium cycling over a 20-year period between two sampling intervals, 1977–1979 (pre- anthracnose) and 1995–2000 (post-anthracnose). Published equations were used to estimate foliar biomass per species for five forest types: alluvial, typic cove, acid cove, oak–hickory, and oak–pine. Calcium concentrations derived from foliage samples were used to estimate annual foliar calcium production per species for understory woody stems (,20 cm dbh) and total foliar calcium production for overstory stems (20 cm dbh). -

Anthracnose Diseases of Dogwood
Agriculture and Natural Resources FSA7564 Anthracnose Diseases of Dogwood Sherrie Smith Introduction to infect flowering dogwood (Cornus Plant Pathologist/Instructor florida), Pacific dogwood (Cornus nut- The most prevalent anthracnose tallii) and, to a lesser extent, Kousa diseases on dogwood in the south- dogwood (Cornus kousa). eastern area of the United States are Spot anthracnose and Dogwood anthracnose. These are two distinct Symptoms infectious plant diseases that should Spot Anthracnose not be confused with each other. Each This type of anthracnose produces disease is caused by a specific micro- small (1-2mm diameter), rounded, pur- organism and produces characteristic ple-bordered spots on the bracts, leaves symptoms in diagnosis. and fruit. Bracts are usually infected first (Figure 1). As with the leaf spots, Spot anthracnose is caused by the fungus Elsinoe corni. Spot anthracnose the centers of the spots on bracts is primarily significant on flowering become light-colored and then drop dogwood (Cornus florida) in Arkansas, out, leaving a “shot-hole” appearance but the fungus can also affect Kousa to the bracts. Infected bracts may dogwood (Cornus kousa). This disease become distorted, smaller or even is common in Arkansas, especially aborted. Leaf distortion can also following frequent rainfall and cool occur as a result of foliar infections. temperatures in April and May. Spot Spots tend to be more numerous in anthracnose is considered a “cosmetic” leaf crevices where water may collect disease and does not usually have an (Figure 2). Spot anthracnose does adverse impact on the overall health not cause a dieback of branches, but of the tree. Dogwood anthracnose is caused by the fungus Discula destructiva and is more serious than Spot anthracnose.