First Insight Into Nutrition Effect on Spawning and Larvae Rearing of the Clam Ruditapes Decussatus L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pathogens and Parasites of the Mussels Mytilus Galloprovincialis and Perna Canaliculus: Assessment of the Threats Faced by New Zealand Aquaculture
Pathogens and Parasites of the Mussels Mytilus galloprovincialis and Perna canaliculus: Assessment of the Threats Faced by New Zealand Aquaculture Cawthron Report No. 1334 December 2007 EXECUTIVE SUMMARY The literature on pathogens and parasites of the mytilid genera Mytilus and Perna was surveyed with particular focus on M. galloprovincialis and P. canaliculus. Likely pathological threats posed to New Zealand mussel aquaculture were identified and recommendations were discussed under the following topics. Epidemiological differences between New Zealand Mytilus and Perna There is a paucity of data for this comparison. Comparability or otherwise would inform predictions as to the threat posed by overseas Mytilus parasites to New Zealand Perna and vice versa. Samples of P. canaliculus and M. galloprovincialis should be surveyed to establish any significant difference in parasite loads or pathology. Invasive dynamics and possible development of pathological threat The greatest potential threat to New Zealand Perna canaliculus aquaculture appears to be posed by parasites introduced by invading Mytilus species. These common ship-borne fouling organisms are a likely source of overseas pathogens, the most important of which are probably Marteilia spp. and disseminated haemic neoplasia. Hybridisation of invasive and local mussels presents a further potential pathology hazard by production of a more susceptible reservoir host thus giving the potential for production of more infected hosts and greater water load of transmission stages. Such an increase in transmission stages might be a cause of concern for Perna canaliculus whose susceptibility is currently unknown. Studies on Marteilia and haemic neoplasia are required to address the following questions: Is Perna canaliculus susceptible to either of these pathogens? What are the current prevalences of these pathogens in local mytilids? What species of mussels are entering New Zealand waters? Do they include Mytilus spp. -

WO 2018/117868 Al 28 June 2018 (28.06.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/117868 Al 28 June 2018 (28.06.2018) W !P O PCT (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A23L 33/18 (2016.01) A61K 35/612 (2015.01) kind of national protection available): AE, AG, AL, AM, C12P 21/06 (2006.01) A61K 35/616 (2015.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, C12P 7/64 (2006 .0 1) A 61K 35/618 (2015.01) CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, A61K 35/00 (2006.01) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, (21) International Application Number: KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, PCT/NZ20 17/050 167 MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (22) International Filing Date: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 20 December 2017 (20.12.2017) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY,TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, 727786 20 December 2016 (20.12.2016) NZ UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (71) Applicant: SANFORD LIMITED [NZ/NZ]; 22 Jellicoe EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, Street, Freemans Bay, Auckland, 1010 (NZ). -

Impacts of the Invasive Ascidian Didemnum Vexillum on Green-Lipped Mussel Perna Canaliculus Aquaculture in New Zealand
Vol. 4: 17–30, 2013 AQUACULTURE ENVIRONMENT INTERACTIONS Published online June 18 doi: 10.3354/aei00069 Aquacult Environ Interact OPENPEN ACCESSCCESS Impacts of the invasive ascidian Didemnum vexillum on green-lipped mussel Perna canaliculus aquaculture in New Zealand Lauren M. Fletcher1,2,*, Barrie M. Forrest1, James J. Bell2 1Cawthron Institute, Private Bag 2, Nelson 7010, New Zealand 2School of Biological Sciences, Victoria University of Wellington, PO Box 600, Wellington 6140, New Zealand ABSTRACT: Biofouling can pose a significant threat to shellfish aquaculture, as fouling organisms are often strong spatial competitors that are able to reach high densities or biomass in relatively short time frames. This study investigated the potential impacts of the colonial ascidian Didem- num vexillum on cultured New Zealand green-lipped mussels Perna canaliculus at one farm in the Marlborough Sounds region. Three size classes of mussels were examined: small (20 to 40 mm shell length at deployment), medium (40 to 60 mm), and large (60 to 70 mm). Replicate 4 m mussel lines were assigned to 1 of 3 treatments: (1) ambient fouling, (2) fouling enhanced by D. vexillum fragment inoculation (in addition to ambient fouling), or (3) control lines that were kept free of D. vexillum. After 15 mo, subsections of lines (0.5 m length) were processed to determine the effects of fouling cover on mussel density within lines, as well as on individual mussel size and condition. A highly significant negative relationship was shown between D. vexillum biomass and mussel density for small mussels, and to a lesser extent for medium mussels. Values of mussel condition indices were similar across size classes and across fouling treatments within each size class. -

Animal Health Import Requirements for Aquatic Animals for Ornamental
RIG.AQ.ON.OUT.19* REPÚBLICA FEDERATIVA DO BRASIL MINISTÉRIO DA AGRICULTURA, PECUÁRIA E ABASTECIMENTO Secretaria de Defesa Agropecuária - SDA Departamento de Saúde Animal e Insumos Pecuários- DSA Coordenação do Trânsito e Quarentena Animal – CTQA REQUISITOS SANITÁRIOS PARA IMPORTAÇÃO DE ANIMAIS AQUÁTICOS PARA FINS ORNAMENTAIS E NÃO DESTINADOS À REPRODUÇÃO COMERCIAL / ANIMAL HEALTH IMPORT REQUIREMENTS FOR AQUATIC ANIMALS FOR ORNAMENTAL PURPOSES NOT INTENDED FOR COMMERCIAL REPRODUCTION 1. Os animais deverão vir acompanhados de Certificado Veterinário Internacional, emitido ou endossado por Veterinário da Autoridade Veterinária do país exportador, em português e na língua oficial do país exportador, contendo:1 The animals must be followed by an International Veterinary Certificate issued or endorsed by a Veterinary of the Veterinary Authority of the exporting country, in Portuguese and in the official language of the exporting country, containing: *Código alfanuméricopara*Código uso MAPA/interno do I. IDENTIFICAÇÃO / IDENTIFICATION a) Nome científico de cada espécie / Scientific name of each species; b) Família a que pertence cada espécie / Taxonomic family of each species; c) Quantidade de cada espécie / Quantity of each species; d) Meio de transporte / Mean of transportation. II. ORIGEM / ORIGIN a) Nome do país exportador / Name of the exporting country; b) Nome do país de origem (se diferente do exportador) / Name of country of origin of the animals (if different from exporter); c) Nome e endereço do estabelecimento exportador / Name and address of exporter; d) Nome e endereço do estabelecimento de origem (se diferente do exportador) / Name and address of origin premise (if different from exporter). Alphanumeric forcode internal use III. DESTINO / DESTINATION III.a. -
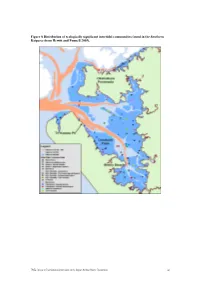
Figure 8 Distribution of Ecologically Significant Intertidal Communities Found in the Southern Kaipara (From Hewitt and Funnell 2005)
Figure 8 Distribution of ecologically significant intertidal communities found in the Southern Kaipara (from Hewitt and Funnell 2005). TP354: Review of Environmental Information on the Kaipara Harbour Marine Environment 21 Figure 9 Interpolated plots of the distribution of total numbers of individuals (A), number of taxa (B), and number of orders (C) found in the cores taken from the intertidal sites (from Hewitt and Funnell 2005). A B C TP354: Review of Environmental Information on the Kaipara Harbour Marine Environment 22 Figure 10 Distribution of subtidal epibenthic habitats found in the Southern Kaipara (from Hewitt and Funnell 2005). TP354: Review of Environmental Information on the Kaipara Harbour Marine Environment 23 Figure 11 Distribution of ecologically significant subtidal communities found in the Southern Kaipara (from Hewitt and Funnell 2005). TP354: Review of Environmental Information on the Kaipara Harbour Marine Environment 24 Figure 12 Interpolated plots of the distribution of total numbers of individuals (A), number of taxa (B), and number of orders (C) found in grabs taken from the subtidal sites (from Hewitt and Funnell 2005). A B C TP354: Review of Environmental Information on the Kaipara Harbour Marine Environment 25 3.2.2 Northern Kaipara Harbour The intertidal and subtidal areas of the northern Kaipara are influenced by several relatively large rivers including the Arapaoa River, Otamatea River, Oruawharo River and Wairoa River (Figure 1). Compared to the southern Kaipara, the northern Kaipara has been studied in far less spatial detail. Several studies have focused on benthic communities within discrete locations (e.g. the Otamatea River; see Robertson et al. -

Executive Summary
EXECUTIVE SUMMARY The New Zealand aquaculture industry is presently dominated by the production of Greenshell™ mussels in terms of total yield, water-space utilisation and total revenue. However, there has been recent emphasis by a number of mussel farmers in the Marlborough Sounds to amend permits and consents so that other bivalve species can be farmed. In response to this, the Ministry of Fisheries commissioned the Cawthron Institute to use available information to perform a desktop investigation into the marginal differences between the environmental interactions of a range of bivalve species to underpin stocking density guidelines. A hazard assessment was used to identify the major environmental interactions between bivalves and the surrounding marine environment and this highlighted several major risk pathways, several of which were through the feeding and excretory behaviour of the bivalve crop. Marginal differences between the transfer of material by the different species were therefore investigated using a range of feeding models and environmental data from the Marlborough Sounds and Glenhaven Aquaculture Centre. The key result from analysis of the marginal differences between a range of bivalve species was that mussels generally appear to exhibit the highest clearance and excretion rates of the bivalves considered. Similarly, biodeposition intensity greater than 400 g/day/1000ind occurred most frequently in mussels (40%) followed by, scallops (33%), cupped oysters (29%), flat oysters (11%), and finally clams/cockles (6%). Overall, it appears that based on the model utilised here, the substitution of mussels, specifically Perna canaliculus, with any of the other alternate species/groups proposed would not be likely to increase either the clearance of the surrounding water, the biodeposition of suspended matter or the amount of dissolved ammonia through excretion. -

Larval Cryopreservation As New Management Tool for Threatened Clam Fisheries
www.nature.com/scientificreports OPEN Larval cryopreservation as new management tool for threatened clam fsheries P. Heres, J. Troncoso & E. Paredes* Cryopreservation is the only reliable method for long-term storage of biological material that guarantees genetic stability. This technique can be extremely useful for the conservation of endangered species and restock natural populations for declining species. Many factors have negatively afected the populations of high economical value shellfsh in Spain and, as a result, many are declining or threatened nowadays. This study was focused on early-life stages of Venerupis corrugata, Ruditapes decussatus and Ruditapes philippinarum to develop successful protocols to enhance the conservation efort and sustainable shellfshery resources. Firstly, common cryoprotecting agents (CPAs) were tested to select the suitable permeable CPA attending to toxicity. Cryopreservation success using diferent combinations of CPA solutions, increasing equilibrium times and larval stages was evaluated attending to survival and shell growth at 2 days post-thawing. Older clam development stages were more tolerant to CPA toxicity, being ethylene- glycol (EG) and Propylene-glycol (PG) the least toxic CPAs. CPA solution containing EG yielded the highest post-thawing survival rate and the increase of equilibration time was not benefcial for clam larvae. Cryopreservation of trochophores yielded around 50% survivorship, whereas over 80% of cryopreserved D-larvae were able to recover after thawing. Te Japanese carpet shell (Ruditapes philippinarum (Adams & Reeve, 1850)), the grooved carpet shell (Ruditapes decussatus (Linnaeus, 1758)) and the slug carpet shell (Venerupis corrugata (Gmelin, 1971)) belong to phylum Mollusca, class Bivalvia, which comprises around 20,000 aquatic and marine species. Tese clams are laterally comprised with two lateral valves. -

Characterisation of Neurons in the Pedal Ganglia of the Green-Lipped
Characterisation of neurons in the pedal ganglia of the green-lipped mussel, Perna canaliculus, using antibodies raised against neuropeptides and neurotransmitters involved in gastropod egg-laying behaviour and bivalve reproduction and spawning Item Type article Authors Mahmud, S.; Mladenov, P.V.; Kaspar, H.; Chakraborty, S.C. Download date 28/09/2021 04:01:29 Link to Item http://hdl.handle.net/1834/33231 Bangladesh]. Fish. Res., 12(1), 2008: 53-60 Characterisation of neurons in the pedal ganglia of the green-lipped mussel, Perna canaliculus, using antibodies raised against neuropeptides and neurotransmitters involved in gastropod egg-laying behaviour and bivalve reproduction and spawning 1 3 S. Mahmud*· , P.V. Mladenov, H. Kaspar and S.C. Chakraborty Department of Marine Science, University of Otago, P.O. Box 56, Dunedin, New Zealand 2Cawthron Institute, Private Bag 2, Nelson, New Zealand 3Department of Fisheries Technology, Bangladesh Agricultural University, Mymensingh 2202 *Corresponding author, present address: Examination Section, Chittagong University of Veterinary and Animal Sciences, Chittagong, Bangladesh Abstract To characterise central neurons in the pedal ganglia of both male and female green lipped mussel, Perna canaliculus immunohistochemical techniques were used. Mollusc antibodies were used against neuropeptides and neurotansmitters known to control reproduction and spawning. Anti-ELH and anti-APGWamide showed very strong immunoreactivity in small type of neurons. Anti-5-HT and anti-DA immunoreactivity was mostly in large type of neurons. The labelled neurons are consistent with descriptions of neurosecretory cells implicated in the control of reproduction and spawning on the basis of earlier histological staining techniques used in this species. The use of selective immunological markers for peptides and amines appears to be a, promising tool for further characterisation of neurosecretory cells, and to isolate an'tl characterise neuropeptides and other biologically active materials involved in the control of reproduction in Perna canaliculus. -

Aquaculture Environment Interactions 11:291
Vol. 11: 291–304, 2019 AQUACULTURE ENVIRONMENT INTERACTIONS Published June 27 https://doi.org/10.3354/aei00314 Aquacult Environ Interact OPENPEN ACCESSCCESS REVIEW Disease threats to farmed green-lipped mussels Perna canaliculus in New Zealand: review of challenges in risk assessment and pathway analysis A. Castinel1,4, S. C. Webb1,*, J. B. Jones2, E. J. Peeler3, B. M. Forrest1 1Cawthron Institute, Nelson 7010, New Zealand 2Murdoch University, Murdoch, Western Australia 6150, Australia 3Centre for Aquaculture Fisheries and the Environment, Weymouth DT4 8UB, UK 4Present address: PO Box 5142, Nelson 7010, New Zealand ABSTRACT: The endemic green-lipped mussel (GLM) Perna canaliculus is a key cultural and economic species for New Zealand. Unlike other cultured shellfish species, GLMs have experi- enced relatively few disease issues. The apparent absence of diseases in both wild and farmed GLM populations does not preclude risks from environmental changes or from the introduction of overseas mussel pathogens and parasites. Potential for disease exchange between the GLM and other mytilid species present in New Zealand has yet to be elucidated. After reviewing and dis- cussing relevant scientific literature, we present an initial assessment of GLM vulnerability to dis- ease threats and the potential risk pathways for mussel pathogens and parasites into New Zealand and highlight a number of challenges. These include knowledge gaps relevant to GLM suscepti- bility to exotic pathogens and parasites, risk pathways into New Zealand and biosecurity risk associated with domestic pathways. Considerations and findings could potentially apply to other farmed aquatic species with limited distribution range and/or low disease exposure. KEY WORDS: Mussel · Disease · Shellfish · Risk pathways · Risk management · Biofouling 1. -

Shelled Molluscs - Berthou P., Poutiers J.-M., Goulletquer P., Dao J.C
FISHERIES AND AQUACULTURE – Vol. II - Shelled Molluscs - Berthou P., Poutiers J.-M., Goulletquer P., Dao J.C. SHELLED MOLLUSCS Berthou P. Institut Français de Recherche pour l'Exploitation de la Mer, Plouzané, France Poutiers J.-M. Muséum National d’Histoire Naturelle, Paris, France Goulletquer P. Institut Français de Recherche pour l'Exploitation de la Mer, La Tremblade, France Dao J.C. Institut Français de Recherche pour l'Exploitation de la Mer, Plouzané, France Keywords: bivalves, gastropods, fisheries, aquaculture, biology, fishing gears, management Contents 1. Introduction 1.1. Uses of Shellfish: An Overview 1.2. Production 2. Species and Fisheries 2.1. Diversity of Species 2.1.1. Edible Species 2.1.2. Shellfish Species Not Used as Food 2.2. Shelled Molluscs Fisheries 2.2.1. Gastropods 2.2.2. Oysters 2.2.3. Mussels 2.2.4. Scallops 2.2.5. Clams 2.3. Shelled Molluscs Cultivation 2.3.1. Gastropods 2.3.2. Oysters 2.3.3. Mussels 2.3.4. ScallopsUNESCO – EOLSS 2.3.5. Clams 3. Harvesting andSAMPLE Cultivation Techniques CHAPTERS 3.1. Harvesting 3.2. Cultivation techniques 4. Biology 4.1. General Ecology 4.2. Growth 4.3. Reproduction 4.4. Larval Stage in Relation to Dispersal and Stock Abundance 4.5. Migration 5. Stock Assessment and Management Approaches ©Encyclopedia of Life Support Systems (EOLSS) FISHERIES AND AQUACULTURE – Vol. II - Shelled Molluscs - Berthou P., Poutiers J.-M., Goulletquer P., Dao J.C. 5.1. Stock Assessment 5.2. Management Strategies 6. Issues for the Future Bibliography Biographical Sketches Summary Shelled molluscs are comprised of bivalves and gastropods. -

Mrna-Seq and Microarray Development for the Grooved Carpet
Leite et al. BMC Genomics 2013, 14:741 http://www.biomedcentral.com/1471-2164/14/741 RESEARCH ARTICLE Open Access mRNA-Seq and microarray development for the Grooved carpet shell clam, Ruditapes decussatus:a functional approach to unravel host -parasite interaction Ricardo B Leite1,8*†, Massimo Milan2†, Alessandro Coppe3, Stefania Bortoluzzi3, António dos Anjos1,4, Richard Reinhardt5, Carlos Saavedra6, Tomaso Patarnello2, M Leonor Cancela1,7 and Luca Bargelloni2 Abstract Background: The Grooved Carpet shell clam Ruditapes decussatus is the autochthonous European clam and the most appreciated from a gastronomic and economic point of view. The production is in decline due to several factors such as Perkinsiosis and habitat invasion and competition by the introduced exotic species, the manila clam Ruditapes philippinarum. After we sequenced R. decussatus transcriptome we have designed an oligo microarray capable of contributing to provide some clues on molecular response of the clam to Perkinsiosis. Results: A database consisting of 41,119 unique transcripts was constructed, of which 12,479 (30.3%) were annotated by similarity. An oligo-DNA microarray platform was then designed and applied to profile gene expression in R. decussatus heavily infected by Perkinsus olseni. Functional annotation of differentially expressed genes between those two conditions- was performed by gene set enrichment analysis. As expected, microarrays unveil genes related with stress/infectious agents such as hydrolases, proteases and others. The extensive role of innate immune system was also analyzed and effect of parasitosis upon expression of important molecules such as lectins reviewed. Conclusions: This study represents a firstattempttocharacterizeRuditapes decussatus transcriptome, an important marine resource for the European aquaculture. -

View the Complete Recommendations List
This document and all information included within it have been produced by the Seafood Watch® program, which is owned and operated by the Monterey Bay Aquarium. The Aquarium holds a registered trademark on the name Seafood Watch. All research, findings, conclusions, ratings, opinions, and analyses produced by the Seafood Watch program—including those appearing within this document—are protected by federal copyright law. All such information is and remains the exclusive property of the Monterey Bay Aquarium. The Aquarium disseminates research, ratings, and other information produced by Seafood Watch to a variety of industry partners. The Aquarium thereby grants to recipients a limited, nontransferable license to possess and use both the Aquarium’s Seafood Watch trademark and its copyright ed material only for internal uses preappro ved in writing by the Aquarium. The Aquarium reserves the right to revoke these licenses at any time and/or to require recipients to sign a written lice nsing agreement on terms defined by the Aquarium. By grantin g these lice nses, the Aquarium does not waive any protections afforded by federal or state intellectual property law. Recipients may use the Aquarium's Seafood Watch materials only in a manner consistent with feder al intellectual property law: o Recipients must advise third parties that Seafood Watch is a registered trademark of the Aquarium that may not be used without the Aquarium's consent. Any use of the Aquarium’s trademark on any printed or digital material—including, but not limited to, on websites and in social media—must include the foll owing statement in a prominent location, in no less than 10-point type: “Seafood Watch is a registered trademark of the Monterey Bay Aquarium.” o Recipients must also include an appropriate copyright notice – e.g., “© 2020, Monterey Bay Aquarium” – on any print or digital reproduction or further dissemination of any Seafood Watch materials.