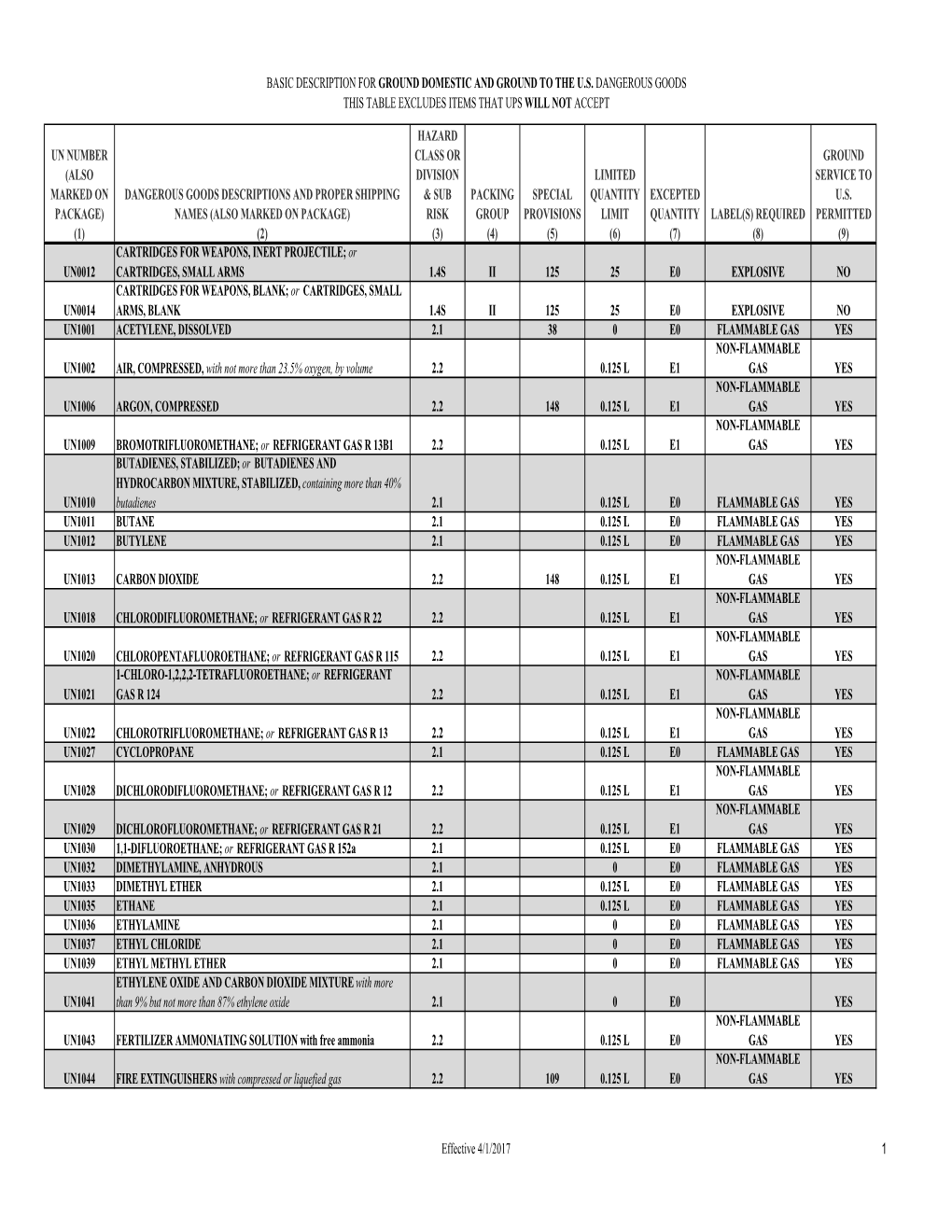
Basic Description for Ground Domestic and Ground to the U.S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Used at Rocky Flats
. TASK 1 REPORT (Rl) IDENTIFICATION OF CHEMICALS AND RADIONUCLIDES USED AT ROCKY FLATS I PROJECT BACKGROUND ChemRisk is conducting a Rocky Flats Toxicologic Review and Dose Reconstruction study for The Colorado Department of Health. The two year study will be completed by the fall of 1992. The ChemRisk study is composed of twelve tasks that represent the first phase of an independent investigation of off-site health risks associated with the operation of the Rocky Flats nuclear weapons plant northwest of Denver. The first eight tasks address the collection of historic information on operations and releases and a detailed dose reconstruction analysis. Tasks 9 through 12 address the compilation of information and communication of the results of the study. Task 1 will involve the creation of an inventory of chemicals and radionuclides that have been present at Rocky Flats. Using this inventory, chemicals and radionuclides of concern will be selected under Task 2, based on such factors as the relative toxicity of the materials, quantities used, how the materials might have been released into the environment, and the likelihood for transport of the materials off-site. An historical activities profile of the plant will be constructed under Task 3. Tasks 4, 5, and 6 will address the identification of where in the facility activities took place, how much of the materials of concern were released to the environment, and where these materials went after the releases. Task 7 addresses historic land-use in the vicinity of the plant and the location of off-site populations potentially affected by releases from Rocky Flats. -

2110 Wxx J V
View Article Online / Journal Homepage / Table of Contents for this issue 2110 BALL AND ABRAM : BISMUTHINITRLTES. Published on 01 January 1913. Downloaded by Cornell University Library 30/06/2017 07:42:59. WXXJ V. --€?ismu t h initrit es. By WALTERCRAVEN BALL and HAROLDHELLING ABRAM. IN previous communications (T., 1905, 87, 761; 1909, 95, 2126; 1910, 97, 1408) one of the authors of the present paper has described several compounds of bismuth nitrite with the alkaline nitrites. These compounds were chiefly remarkable aa affording a method for the gravimetric estimation of sodium, and for its separation from potmsium, owing to the insolubility of msium sodium bismuthinitrite and the non-formation of the corresponding potassium salt (T., 1910, 97, 1408). As it is unusual to find such sharp differences in the behaviour of sodium and potassium salts, the present authors have investigated all the salts of this series which they have been able to obtain, in order to discover, if possible, other facts bearing on this difference in behaviour. The salts which have been obtained all fall into two groups, of which View Article Online BALL AND ABKAM : BlSMUTElINITRlTES. 2111 the general formula are respectively X,Bi(NO,), and X,YBi(N02),. In these formulae X represents any of the metals ammonium, potassium, rubidium, msium, and thallium, whilst Y stands for either lithium, sodium, or silver. There are thus possible five compounds of the X,Bi(NO,), series, or simple bismuthinitrites, and of these, four have been obtained, the attempts to obtain the ammonium salt having been 60 far unsuccessful owing to the great instability of concentrated solutions of ammonium nitrite in the presence of acid. -

Underactive Thyroid
Underactive Thyroid PDF generated using the open source mwlib toolkit. See http://code.pediapress.com/ for more information. PDF generated at: Thu, 21 Jun 2012 14:27:58 UTC Contents Articles Thyroid 1 Hypothyroidism 14 Nutrition 22 B vitamins 47 Vitamin E 53 Iodine 60 Selenium 75 Omega-6 fatty acid 90 Borage 94 Tyrosine 97 Phytotherapy 103 Fucus vesiculosus 107 Commiphora wightii 110 Nori 112 Desiccated thyroid extract 116 References Article Sources and Contributors 121 Image Sources, Licenses and Contributors 124 Article Licenses License 126 Thyroid 1 Thyroid thyroid Thyroid and parathyroid. Latin glandula thyroidea [1] Gray's subject #272 1269 System Endocrine system Precursor Thyroid diverticulum (an extension of endoderm into 2nd Branchial arch) [2] MeSH Thyroid+Gland [3] Dorlands/Elsevier Thyroid gland The thyroid gland or simply, the thyroid /ˈθaɪrɔɪd/, in vertebrate anatomy, is one of the largest endocrine glands. The thyroid gland is found in the neck, below the thyroid cartilage (which forms the laryngeal prominence, or "Adam's apple"). The isthmus (the bridge between the two lobes of the thyroid) is located inferior to the cricoid cartilage. The thyroid gland controls how quickly the body uses energy, makes proteins, and controls how sensitive the body is to other hormones. It participates in these processes by producing thyroid hormones, the principal ones being triiodothyronine (T ) and thyroxine which can sometimes be referred to as tetraiodothyronine (T ). These hormones 3 4 regulate the rate of metabolism and affect the growth and rate of function of many other systems in the body. T and 3 T are synthesized from both iodine and tyrosine. -

Some Physical Properties of Ionic Melts in Relation to Their Structure
1. SOME PHYSICAL PROPERTIES OF IONIC MELTS IN RELATION TO THEIR STRUCTURE By William Ewen Smith, B.Sc., A.R.C.S.T. A Thesis submitted to the University of London for the Degree of Doctor of Philosophy. Department of Chemical Engineering and Chemical Technology, Imperial College of Science and Technology, London, S.W.7. October 12§.2. 2. Abstract. The unusual physical behaviour of the melt of potassium nitrate and calcium nitrate had previously been reported. In the present study, the liquid behaviour is defined quantitatively in terms of physical properties, some results are taken on related systems and a structural explanation postulated to explain the data. A brief review of fundamental liquid theory and theories of viscosity transport are given. A more detailed analysis of the experimental measurements in molten salts most relevant to a structural analysis and a study of previous work in the monodivalent nitrate melts leads to a simple structural model capable of a limited interpre- tation of experimental behaviour. Qualitative studies on the nature of the low temperature form of certain compositions of the mixed nitrate melts verifies previous kreports of glass forma- tion. A quantitative study in the liquid range of the viscosity of the binary mixtvres of sodium, potassium, rubidium and caesium nitrate with calcium nitrate estab- lished the essentially non-Arhenius nature of the viscosity plots. An investigation of the electrical conductivity and 3. U.V. spectra of the melts was then carried out. The con- clusions drawn indicated the possibility of anomalous dielectric effects in the supercooled melt. Techniques were developed for this study and results indicatiag some form of loss mechanism obtained. -

Basic Description for Ground and Air Hazardous
BASIC DESCRIPTION FOR GROUND AND AIR GROUND AND AIR HAZARDOUS MATERIALS SHIPMENTS GROUND SHIPMENTS AIR SHIPMENTS SHIPMENTS HAZARD DOT DOT CLASS OR MAXIMUM EXEMPTION, GROUND EXEMPTION, HAZARDOUS MATERIALS DESCRIPTIONS DIVISION I.D. NUMBER LABEL(S) REQUIRED OR QUANTITY PER SPECIAL SERVICE TO LABEL(S) REQUIRED OR MAXIMUM NET CARGO SPECIAL NON-BULK AND PROPER SHIPPING NAME (Subsidiary if (ALSO MARK PACKING EXEMPTION, SPECIAL PERMIT INNER PERMIT CANADA EXEMPTION, SPECIAL PERMIT QUANTITY PER AIRCRAFT PERMIT SPECIAL EXCEPTIONS PACKAGING (ALSO MARK ON PACKAGE) applicable) ON PACKAGE) GROUP OR EXCEPTION RECEPTACLE OR 173.13 PERMITTED OR EXCEPTION PACKAGE** QUANTITY OR 173.13 PROVISIONS §173.*** §173.*** (1) (2) (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) (13) (14) (15) Accellerene, see p-Nitrosodimethylaniline Accumulators, electric, see Batteries, wet etc Accumulators, pressurized, pneumatic or hydraulic (containing non-flammable gas), see Articles pressurized, pneumatic or hydraulic (containing non-flammable gas) FLAMMABLE FLAMMABLE Acetal 3 UN1088 II LIQUID * YES LIQUID * 5 L 150 202 FLAMMABLE Acetaldehyde 3 UN1089 I LIQUID YES Forbidden None 201 May not be regulated when shipped via UPS Acetaldehyde ammonia 9 UN1841 III ground YES CLASS 9 * 30 kg 30 kg 155 204 FLAMMABLE FLAMMABLE Acetaldehyde oxime 3 UN2332 III LIQUID * YES LIQUID * 25 L 150 203 CORROSIVE, CORROSIVE, Acetic acid, glacial or Acetic acid solution, FLAMMABLE FLAMMABLE A3, A6, with more than 80 percent acid, by mass 8 (3) UN2789 II LIQUID * YES LIQUID * 1 L A7, A10 154 202 Acetic -

Table of Contents
UNIVERSITY OF MISSOURI-KANSAS CITY CHEMICAL MANAGEMENT PLAN Revised May 2016 UMKC CHEMICAL MANAGEMENT PLAN This document constitutes the Chemical Management Plan (CMP) for the University of Missouri-Kansas City (UMKC). It was developed by the Environmental Health and Safety Department (EHS), to ensure the safe and proper use of hazardous and non- hazardous chemicals and to comply with applicable governmental regulations addressing the disposal of these chemicals. In addition, it was developed to foster waste minimization, and to provide the faculty and the staff with a management program to reduce the potential for accidents involving hazardous chemicals and/or wastes. Elements of the CMP include: a. a procedure for identifying potential or actual hazardous chemicals or wastes b. a procedure for periodic reexamination of those hazardous chemicals or wastes identified by the procedure in (a.) above as well as a systematic method for identification and evaluation of any new potential or actual hazardous chemicals or wastes c. procedures for labeling, and inventorying hazardous chemicals or wastes d. a procedure for identification and training of personnel directly responsible for ensuring that (a.), (b.), and (c.) are implemented e. a procedure for monitoring, recording, and reporting compliance with the CMP f. a procedure by which information generated by the CMP is provided to the persons performing waste analyses Each element is addressed as part of the complete CMP in the following paragraphs. 4 Table of Contents 1 Definitions 7 2 Identification -

Chemical Resistance of Plastics
(c) Bürkle GmbH 2010 Important Important information The tables “Chemical resistance of plastics”, “Plastics and their properties” and “Viscosity of liquids" as well as the information about chemical resistance given in the particular product descriptions have been drawn up based on information provided by various raw material manufacturers. These values are based solely on laboratory tests with raw materials. Plastic components produced from these raw materials are frequently subject to influences that cannot be recognized in laboratory tests (temperature, pressure, material stress, effects of chemicals, construction features, etc.). For this reason the values given are only to be regarded as being guidelines. In critical cases it is essential that a test is carried out first. No legal claims can be derived from this information; nor do we accept any liability for it. A knowledge of the chemical and mechanical Copyright This table has been published and updated by Bürkle GmbH, D-79415 Bad Bellingen as a work of reference. This Copyright clause must not be removed. The table may be freely passed on and copied, provided that Extensions, additions and translations If your own experiences with materials and media could be used to extend this table then we would be pleased to receive any additional information. Please send an E-Mail to [email protected]. We would also like to receive translations into other languages. Please visit our website at http://www.buerkle.de from time to Thanks Our special thanks to Franz Kass ([email protected]), who has completed and extended these lists with great enthusiasm and his excellent specialist knowledge. -

Metal Carbonyls
MODULE 1: METAL CARBONYLS Key words: Carbon monoxide; transition metal complexes; ligand substitution reactions; mononuclear carbonyls; dinuclear carbonyls; polynuclear carbonyls; catalytic activity; Monsanto process; Collman’s reagent; effective atomic number; 18-electron rule V. D. Bhatt / Selected topics in coordination chemistry / 2 MODULE 1: METAL CARBONYLS LECTURE #1 1. INTRODUCTION: Justus von Liebig attempted initial experiments on reaction of carbon monoxide with metals in 1834. However, it was demonstrated later that the compound he claimed to be potassium carbonyl was not a metal carbonyl at all. After the synthesis of [PtCl2(CO)2] and [PtCl2(CO)]2 reported by Schutzenberger (1868) followed by [Ni(CO)4] reported by Mond et al (1890), Hieber prepared numerous compounds containing metal and carbon monoxide. Compounds having at least one bond between carbon and metal are known as organometallic compounds. Metal carbonyls are the transition metal complexes of carbon monoxide containing metal-carbon bond. Lone pair of electrons are available on both carbon and oxygen atoms of carbon monoxide ligand. However, as the carbon atoms donate electrons to the metal, these complexes are named as carbonyls. A variety of such complexes such as mono nuclear, poly nuclear, homoleptic and mixed ligand are known. These compounds are widely studied due to industrial importance, catalytic properties and structural interest. V. D. Bhatt / Selected topics in coordination chemistry / 3 Carbon monoxide is one of the most important π- acceptor ligand. Because of its π- acidity, carbon monoxide can stabilize zero formal oxidation state of metals in carbonyl complexes. 2. SYNTHESIS OF METAL CARBONYLS Following are some of the general methods of preparation of metal carbonyls. -

Approved and Prohibited Fireworks Chemicals
Version 2 - Updated November 5, 2019 Approved and Prohibited Fireworks Chemicals (NOTE: Restriction Column is added to group restrictions together) Chemical Formula Typical Use Restrictions Alloprene (Chlorinated Rubber) Not Required Color Intensifier Ok for use in a break charge and other Aluminum (> 53 microns) Al Fuel compositions including report compositions Aluminum (≤ 53 microns) Al Fuel Report composition only Not to exceed 5% of formulation Ammonium Dichromate (NH ) Cr O Oxygen Donor / Colored Ash 4 2 2 7 Prohibited if mixed with a Chlorate Ammonium Perchlorate NH4ClO4 Oxygen Donor Prohibited if mixed with a Chlorate Anthracene C14H10 Fuel Antimony Sb Fuel Antimony Sulfide: Antimonous Sulfide or Antimony Trisulfide Sb2S3 Fuel Antimony Trioxide Sb2O3 Oxygen Donor Barium Carbonate BaCO3 Color Agent 1) In smoke formulations an equal or greater weight of bicarbonates or carbonates is required; 2) In all other devices the total chemical Barium Chlorate Ba(ClO ) Oxygen Donor / Color Agent 3 2 composition cannot exceed 4 grams of which no more than 15 percent can be chlorate salts; 3) Permitted in firecrackers, party poppers and booby traps. Barium Nitrate Ba(NO3)2 Oxygen Donor / Color Agent Barium Oxalate BaC2O4 Color Agent Barium Phthalate Ba(C8H5O4)2 Whistle / Color Agent Barium Sulfate BaSO4 Oxygen Donor / Color Agent Benzoic Acid Potassium Salt (Potassium Benzoate) KC6H5CO2 or KC7H5O2 Whistle / Fuel Benzoic Acid Sodium Salt (Sodium Benzoate) NaC6H5CO2 or NaC7H5O2 Whistle / Fuel Bismuth Oxide or Bismuth Trioxide Bi2O3 Oxygen Donor -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

2020 Emergency Response Guidebook
2020 A guidebook intended for use by first responders A guidebook intended for use by first responders during the initial phase of a transportation incident during the initial phase of a transportation incident involving hazardous materials/dangerous goods involving hazardous materials/dangerous goods EMERGENCY RESPONSE GUIDEBOOK THIS DOCUMENT SHOULD NOT BE USED TO DETERMINE COMPLIANCE WITH THE HAZARDOUS MATERIALS/ DANGEROUS GOODS REGULATIONS OR 2020 TO CREATE WORKER SAFETY DOCUMENTS EMERGENCY RESPONSE FOR SPECIFIC CHEMICALS GUIDEBOOK NOT FOR SALE This document is intended for distribution free of charge to Public Safety Organizations by the US Department of Transportation and Transport Canada. This copy may not be resold by commercial distributors. https://www.phmsa.dot.gov/hazmat https://www.tc.gc.ca/TDG http://www.sct.gob.mx SHIPPING PAPERS (DOCUMENTS) 24-HOUR EMERGENCY RESPONSE TELEPHONE NUMBERS For the purpose of this guidebook, shipping documents and shipping papers are synonymous. CANADA Shipping papers provide vital information regarding the hazardous materials/dangerous goods to 1. CANUTEC initiate protective actions. A consolidated version of the information found on shipping papers may 1-888-CANUTEC (226-8832) or 613-996-6666 * be found as follows: *666 (STAR 666) cellular (in Canada only) • Road – kept in the cab of a motor vehicle • Rail – kept in possession of a crew member UNITED STATES • Aviation – kept in possession of the pilot or aircraft employees • Marine – kept in a holder on the bridge of a vessel 1. CHEMTREC 1-800-424-9300 Information provided: (in the U.S., Canada and the U.S. Virgin Islands) • 4-digit identification number, UN or NA (go to yellow pages) For calls originating elsewhere: 703-527-3887 * • Proper shipping name (go to blue pages) • Hazard class or division number of material 2.