The Solubility of Some Sparingly Soluble Salts of Zinc and Cadmium in Water and in Aqueous Electrolyte Solutions
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Zinc Fluoride Product Stewardship Summary February 2011
Zinc Fluoride Product Stewardship Summary February 2011 ZnF2 Chemical Name: Zinc Fluoride Chemical Category (if applicable): Metal Halide Synonyms: Zinc difluoride; ZnF2 CAS Number: 7783-49-5 CAS Name: Zinc fluoride EC (EINECS) Number: 232-001-9 Document Number: GPS0041 V1.0 Zinc fluoride (ZnF2) is used in the manufacture of metals, in fluxes, chemical synthesis, and in the manufacture of special glasses.. Exposure can occur at either a ZnF2 manufacturing facility or at other manufacturing, packaging or storage facilities that handle ZnF2. Persons involved in maintenance, sampling and testing activities, or in the loading and unloading of ZnF2 packages are at risk of exposure, but worker exposure can be controlled with the use of proper general mechanical ventilation and personal protective equipment. Workplace exposure limits for fluoride ion have been established for use in worksite safety programs. When ZnF2 is a component of consumer products, users should follow manufacturer’s use and/or label instructions. ZnF2 dusts released to the atmosphere and deposited in soil or surface water in the vicinity of production sites have negligible impact on the environment. Please see the MSDS for additional information. ZnF2 s a nonflammable solid that is stable under normal conditions. Contact of ZnF2 with water or extended skin contact under moist conditions can produce hydrofluoric acid (HF), a very dangerous acid. Breathing ZnF2 dust can irritate the nose, throat, and lungs. Short-term exposure to high concentrations of ZnF2 may cause nausea, vomiting, loss of appetite and weakness. Long-term exposure to ZnF2 may cause deposits of fluorides in bones and teeth, a condition called fluorosis, which may result in pain, disability and discoloration or mottling of teeth. -

Chain-Length-Identification Strategy in Zinc Polyphosphate Glasses by Means of XPS and Tof-SIMS
Research Collection Journal Article Chain-length-identification strategy in zinc polyphosphate glasses by means of XPS and ToF-SIMS Author(s): Crobu, Maura; Rossi, Antonella; Mangolini, Filippo; Spencer, Nicholas D. Publication Date: 2012 Permanent Link: https://doi.org/10.3929/ethz-b-000048615 Originally published in: Analytical and Bioanalytical Chemistry 403(5), http://doi.org/10.1007/s00216-012-5836-7 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Anal Bioanal Chem (2012) 403:1415–1432 DOI 10.1007/s00216-012-5836-7 ORIGINAL PAPER Chain-length-identification strategy in zinc polyphosphate glasses by means of XPS and ToF-SIMS Maura Crobu & Antonella Rossi & Filippo Mangolini & Nicholas D. Spencer Received: 10 September 2011 /Revised: 29 January 2012 /Accepted: 3 February 2012 /Published online: 27 March 2012 # Springer-Verlag 2012 Abstract The surface chemistry of amorphous zinc poly- linked through P–O–P bonds predominate in the metaphos- phosphates of different compositions (ranging from zinc meta- phate composition, while when the zinc content is increased, phosphate to zinc orthophosphate) has been investigated by the chains become shorter, ultimately being replaced by PO4 means of X-ray photoelectron spectroscopy (XPS) and time- monomers in the orthophosphate composition. of-flight secondary-ion mass spectroscopy (ToF-SIMS). The identification of the chain length of zinc polyphosphates by Keywords Polyphosphate glasses . X-ray photoelectron XPS was on the basis of the integrated intensity ratio of the spectroscopy. Time-of-flight secondary-ion mass bridging (P–O–P) and nonbridging (P0OandP–O–M) oxy- spectroscopy. -

Evaluation of Antibacterial and Cytocompatible Properties Of
Dental Materials Journal 2020; : – Evaluation of antibacterial and cytocompatible properties of multiple-ion releasing zinc-fluoride glass nanoparticles Erika NISHIDA1, Hirofumi MIYAJI1, Kanako SHITOMI1, Tsutomu SUGAYA1 and Tsukasa AKASAKA2 1 Department of Periodontology and Endodontology, Faculty of Dental Medicine, Hokkaido University, N13 W 7, Kita-ku, Sapporo, Hokkaido 060- 8586, Japan 2 Department of Biomedical Materials and Engineering, Faculty of Dental Medicine, Hokkaido University, N13 W 7, Kita-ku, Sapporo, Hokkaido 060- 8586, Japan Corresponding author, Hirofumi MIYAJI; E-mail: [email protected] Zinc-fluoride glass nanoparticles (Zinc-F) release several ions, such as fluoride, zinc and calcium ions, through acid-base reactions. The aim of this study was to evaluate the antibacterial and cytotoxic properties of Zinc-F. Antibacterial tests showed that a Zinc-F eluting solution significantly reduced the turbidity and colony-forming units ofStreptococcus mutans and Actinomyces naeslundii, compared to that of calcium-fluoroaluminosilicate glass nanoparticles without zinc ions. In live/dead staining, Zinc-F eluate significantly decreased green-stained bacterial cells, indicating live cells, compared with the control (no application). Human dentin coated with Zinc-F showed suppressed S. mutans and A. naeslundii biofilm formation. Additionally, Zinc-F eluate showed low cytotoxic effects in osteoblastic and fibroblastic cells. Therefore, our findings suggested that Zinc-F exhibits antibacterial and biocompatible properties through multiple-ion release. Keywords: Actinomyces naeslundii, Biocompatibility, Dentin, Streptococcus mutans, Zinc-fluoride glass nanoparticles calcium and silicon and a phosphoric acid solution. It is INTRODUCTION assumed that Zinc-F aggregates on the dentin surface to Root caries has become a major problem in elderly close dentinal tubules and release zinc, fluoride, calcium people with exposed tooth roots caused by aging or and silicon ions through acid-base reactions, such as periodontal disease. -

ZINC SILICOFLUORIDE CAS Number
Common Name: ZINC SILICOFLUORIDE CAS Number: 16871-71-9 RTK Substance number: 2043 DOT Number: UN 2855 Date: December 2001 ------------------------------------------------------------------------- ----------------------------------------------------------------------- HAZARD SUMMARY * Zinc Silicofluoride can affect you when breathed in and * If you think you are experiencing any work-related health by passing through your skin. problems, see a doctor trained to recognize occupational * Contact can irritate the skin and eyes. Prolonged contact diseases. Take this Fact Sheet with you. can cause skin rash and ulcers, and damage to the eyes. * Breathing Zinc Silicofluoride can irritate the nose, throat WORKPLACE EXPOSURE LIMITS and lungs causing coughing, wheezing and/or shortness of The following exposure limits are for Fluoride: (measured as breath. Fluorine): * Very high exposure can cause Fluoride poisoning with stomach pain, weakness, convulsions, collapse and death. OSHA: The legal airborne permissible exposure limit * Repeated high exposure can cause deposits of Fluorides in (PEL) is 2.5 mg/m3 averaged over an 8-hour the bones and teeth, a condition called “Fluorosis.” This workshift. may cause pain, disability and mottling of the teeth. * The above health effects do NOT occur at the level of NIOSH: The recommended airborne exposure limit is Fluoride used in water for preventing cavities in teeth. 2.5 mg/m3 averaged over a 10-hour workshift. * CONSULT THE NEW JERSEY DEPARTMENT OF HEALTH AND SENIOR SERVICES HAZARDOUS ACGIH: The recommended airborne exposure limit is SUBSTANCE FACT SHEET ON FLUORINE. 2.5 mg/m3 averaged over an 8-hour workshift. IDENTIFICATION * The above exposure limits are for air levels only. When Zinc Silicofluoride is a white, sand-like powder. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

United States Patent Office Patented June 25, 1963
3,095,356 United States Patent Office Patented June 25, 1963 1. 2 3,095,356 of fluoride uptake by the teeth. Soluble fluorides are DENTIFRICE COMPRISING INSOLUBLE SODIUM found to be taken up to a greater extent when these metal METAPHOSPHATE AND A CADMUM, TN, saits are also present with the fluoride. The proportion ZINC, MANGANESE OR IRON COMPOUND TO of the fluorides, such as aluminum, tin, or sodium fluoride INHIBIT CALCUM ON SEQUESTERNG employed in the practice of the present invention is in the Henry V. Moss, Clayton, Mo, assignor to Monsanto range of 0.05 to 1% by weight of the dentifrice composi Chemical Company, St. Louis, Mo., a corporation of tion. However, the total proportion of metal compounds Delaware w of the group of cadmium, tin, zinc, manganese, alumi No Drawing. Filed Feb. 20, 1956, Ser. No. 566,353 num and iron which are present in the dentifrice is within 11 Claims. (CI. 167-93) 10 the broader range of 0.05 to 50 weight percent. This invention relates to polishing compositions suitable According to one of the present theories as to the for use as a dentifrice base and particularly to those com molecular aggregation of phosphate salts, the insoluble positions which contain the insoluble form of sodium sodium metaphosphate containing the usual impurities, metaphosphate as an ingredient thereof. may exist as a chain-type of molecular structure, and also An object of the present invention is the provision of 5 in the form of relatively short-chain polyphosphates. Ac an improved identifice. - cording to this theory, the presently employed metal com The use of the so-called insoluble form of sodium pounds control the sequestering action of polyphosphates, metaphosphate in finely divided form has been proposed thereby controlling the type of molecular structures pre as a dentifrice. -

Chemical List
1 EXHIBIT 1 2 CHEMICAL CLASSIFICATION LIST 3 4 1. Pyrophoric Chemicals 5 1.1. Aluminum alkyls: R3Al, R2AlCl, RAlCl2 6 Examples: Et3Al, Et2AlCl, EtAlCl2, Me3Al, Diethylethoxyaluminium 7 1.2. Grignard Reagents: RMgX (R=alkyl, aryl, vinyl X=halogen) 8 1.3. Lithium Reagents: RLi (R = alkyls, aryls, vinyls) 9 Examples: Butyllithium, Isobutyllithium, sec-Butyllithium, tert-Butyllithium, 10 Ethyllithium, Isopropyllithium, Methyllithium, (Trimethylsilyl)methyllithium, 11 Phenyllithium, 2-Thienyllithium, Vinyllithium, Lithium acetylide ethylenediamine 12 complex, Lithium (trimethylsilyl)acetylide, Lithium phenylacetylide 13 1.4. Zinc Alkyl Reagents: RZnX, R2Zn 14 Examples: Et2Zn 15 1.5. Metal carbonyls: Lithium carbonyl, Nickel tetracarbonyl, Dicobalt octacarbonyl 16 1.6. Metal powders (finely divided): Bismuth, Calcium, Cobalt, Hafnium, Iron, 17 Magnesium, Titanium, Uranium, Zinc, Zirconium 18 1.7. Low Valent Metals: Titanium dichloride 19 1.8. Metal hydrides: Potassium Hydride, Sodium hydride, Lithium Aluminum Hydride, 20 Diethylaluminium hydride, Diisobutylaluminum hydride 21 1.9. Nonmetal hydrides: Arsine, Boranes, Diethylarsine, diethylphosphine, Germane, 22 Phosphine, phenylphosphine, Silane, Methanetellurol (CH3TeH) 23 1.10. Non-metal alkyls: R3B, R3P, R3As; Tributylphosphine, Dichloro(methyl)silane 24 1.11. Used hydrogenation catalysts: Raney nickel, Palladium, Platinum 25 1.12. Activated Copper fuel cell catalysts, e.g. Cu/ZnO/Al2O3 26 1.13. Finely Divided Sulfides: Iron Sulfides (FeS, FeS2, Fe3S4), and Potassium Sulfide 27 (K2S) 28 REFERRAL -

Acutely / Extremely Hazardous Waste List
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extemely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extemely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extemely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extemely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extemely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extemely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extemely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extemely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extemely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Attachment 3-1 Guidance for Developing Ecological Soil
Attachment 3-1 Guidance for Developing Ecological Soil Screening Levels (Eco-SSLs) Eco-SSL Standard Operating Procedure (SOP # 1): Plant and Soil Invertebrate Literature Search and Acquisition OSWER Directive 92857-55 November 2003 This page intentionally left blank OVERVIEW Currently, there is a lack of clear guidance in setting terrestrial effect thresholds when conducting risk assessments. Without an EPA-approved, peer-reviewed, ecologically-based terrestrial effect database, the process to develop thresholds is problematic both to EPA, other federal agencies, states, and concerned private parties. Identification of published toxicity studies on invertebrates, microbial processes and plants is a key step in the derivation of benchmarks. The purpose of the Task Group 4, Standard Operating Procedure Number 1: Literature Search and Acquisition (referred to as TG4-SOP#1) is to document procedures used to identify and acquire potentially relevant toxicology literature for use in setting ecological soil screening levels. The literature search strategy is designed to locate worldwide terrestrial toxicity literature that includes the effects of chemicals of concern on terrestrial soil-dwelling invertebrates and plants. The literature acquisition process is designed to ensure timely acquisition of relevant publications. LITERATURE IDENTIFICATION Potentially relevant literature for developing ecological soil screening levels (Eco-SSLs) is identified by examining hard copies of relevant journals, bibliographies and guidance publications and through the use of a comprehensive computerized literature search strategy. These procedures are designed to locate worldwide terrestrial toxicology literature that includes the effects of specific toxic substances with an emphasis on exposure via soil. Paper-based Literature Identification The paper-based literature identification process includes the scanning of relevant review article bibliographies and key journals held in the U.S. -

High Purity Inorganics
High Purity Inorganics www.alfa.com INCLUDING: • Puratronic® High Purity Inorganics • Ultra Dry Anhydrous Materials • REacton® Rare Earth Products www.alfa.com Where Science Meets Service High Purity Inorganics from Alfa Aesar Known worldwide as a leading manufacturer of high purity inorganic compounds, Alfa Aesar produces thousands of distinct materials to exacting standards for research, development and production applications. Custom production and packaging services are part of our regular offering. Our brands are recognized for purity and quality and are backed up by technical and sales teams dedicated to providing the best service. This catalog contains only a selection of our wide range of high purity inorganic materials. Many more products from our full range of over 46,000 items are available in our main catalog or online at www.alfa.com. APPLICATION FOR INORGANICS High Purity Products for Crystal Growth Typically, materials are manufactured to 99.995+% purity levels (metals basis). All materials are manufactured to have suitably low chloride, nitrate, sulfate and water content. Products include: • Lutetium(III) oxide • Niobium(V) oxide • Potassium carbonate • Sodium fluoride • Thulium(III) oxide • Tungsten(VI) oxide About Us GLOBAL INVENTORY The majority of our high purity inorganic compounds and related products are available in research and development quantities from stock. We also supply most products from stock in semi-bulk or bulk quantities. Many are in regular production and are available in bulk for next day shipment. Our experience in manufacturing, sourcing and handling a wide range of products enables us to respond quickly and efficiently to your needs. CUSTOM SYNTHESIS We offer flexible custom manufacturing services with the assurance of quality and confidentiality. -
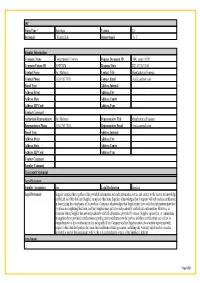
Material Composition Declaration
IPC Form Type * Distribute Version 2.0 Sectionals - MaterialInfo Subsectionals - A- C Supplier Information Company Name Contemporary Controls Request Document ID EISK_series_MCD Company Unique ID 363857474 Response Date 2021-07-30-12:00 Contact Name Neil Maloney Contact Title Manufacturing Engineer Contact Phone (630) 963 7070; Contact Email [email protected] Email Type Address Internal Address Street Address City Address State Address Contry Address ZIP Code Address Type Contact Comment Authorized Representative Neil Maloney Representative Title Manufacturing Engineer Representative Phone (630) 963 7070; Representative Email [email protected] Email Type Address Internal Address Street Address City Address State Address Contry Address ZIP Code Address Type Contact Comment Supplier Comment Uncertainty Statement Legal Statement Supplier Acceptance true Legal Declaration Standard Legal Statement Supplier certifies that it gathered the provided information and such information is true and correct to the best of its knowledge and belief, as of the date that Supplier completes this form. Supplier acknowledges that Company will rely on this certification in determining the compliance of its products. Company acknowledges that Supplier may have relied on information provided by others in completing this form, and that Supplier may not have independently verified such information. However, in situations where Supplier has not independently verified information provided by others, Supplier agrees that, at a minimum, its suppliers have provided certifications regarding their contributions to the part(s), and those certifications are at least as comprehensive as the certification in this paragraph. If the Company and the Supplier enter into a written agreement with respect to the identified part(s), the terms and conditions of that agreement, including any warranty rights and/or remedies provided as part of that agreement, will be the sole and exclusive source of the Supplier’s liability Attachment . -

20210311 IAEG AD-DSL V5.0 for Pdf.Xlsx
IAEGTM AD-DSL Release Version 4.1 12-30-2020 Authority: IAEG Identity: AD-DSL Version number: 4.1 Issue Date: 2020-12-30 Key Yellow shading indicates AD-DSL family group entries, which can be expanded to display a non-exhaustive list of secondary CAS numbers belonging to the family group Substance Identification Change Log IAEG Regulatory Date First Parent Group IAEG ID CAS EC Name Synonyms Revision Date ECHA ID Entry Type Criteria Added IAEG ID IAEG000001 1327-53-3 215-481-4 Diarsenic trioxide Arsenic trioxide R1;R2;D1 2015-03-17 2015-03-17 100.014.075 Substance Direct Entry IAEG000002 1303-28-2 215-116-9 Diarsenic pentaoxide Arsenic pentoxide; Arsenic oxide R1;R2;D1 2015-03-17 2015-03-17 100.013.743 Substance Direct Entry IAEG000003 15606-95-8 427-700-2 Triethyl arsenate R1;R2;D1 2015-03-17 2017-08-14 100.102.611 Substance Direct Entry IAEG000004 7778-39-4 231-901-9 Arsenic acid R1;R2;D1 2015-03-17 2015-03-17 100.029.001 Substance Direct Entry IAEG000005 3687-31-8 222-979-5 Trilead diarsenate R1;R2;D1 2015-03-17 2017-08-14 100.020.890 Substance Direct Entry IAEG000006 7778-44-1 231-904-5 Calcium arsenate R1;R2;D1 2015-03-17 2017-08-14 100.029.003 Substance Direct Entry IAEG000009 12006-15-4 234-484-1 Cadmium arsenide Tricadmium diarsenide R1;R2;D1 2017-08-14 2017-08-14 Substance Direct Entry IAEG000021 7440-41-7 231-150-7 Beryllium (Be) R2 2015-03-17 2019-01-24 Substance Direct Entry IAEG000022 1306-19-0 215-146-2 Cadmium oxide R1;R2;D1 2015-03-17 2017-08-14 100.013.770 Substance Direct Entry IAEG000023 10108-64-2 233-296-7 Cadmium