365 Letter to the Editor Carcinoid of the Minor Duodenal Papilla Associated with Pancreas Divisum: Case Report and Review Of
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
Anatomy of Small Intestine Doctors Notes Notes/Extra Explanation Please View Our Editing File Before Studying This Lecture to Check for Any Changes
Color Code Important Anatomy of Small Intestine Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives: At the end of the lecture, students should: List the different parts of small intestine. Describe the anatomy of duodenum, jejunum & ileum regarding: the shape, length, site of beginning & termination, peritoneal covering, arterial supply & lymphatic drainage. Differentiate between each part of duodenum regarding the length, level & relations. Differentiate between the jejunum & ileum regarding the characteristic anatomical features of each of them. Abdomen What is Mesentery? It is a double layer attach the intestine to abdominal wall. If it has mesentery it is freely moveable. L= liver, S=Spleen, SI=Small Intestine, AC=Ascending Colon, TC=Transverse Colon Abdomen The small intestines consist of two parts: 1- fixed part (no mesentery) (retroperitoneal) : duodenum 2- free (movable) part (with mesentery) :jejunum & ileum Only on the boys’ slides RELATION BETWEEN EMBRYOLOGICAL ORIGIN & ARTERIAL SUPPLY مهم :Extra Arterial supply depends on the embryological origin : Foregut Coeliac trunk Midgut superior mesenteric Hindgut Inferior mesenteric Duodenum: • Origin: foregut & midgut • Arterial supply: 1. Coeliac trunk (artery of foregut) 2. Superior mesenteric: (artery of midgut) The duodenum has 2 arterial supply because of the double origin The junction of foregut and midgut is at the second part of the duodenum Jejunum & ileum: • Origin: midgut • Arterial -

Small & Large Intestine
Small & Large Intestine Gastrointestinal block-Anatomy-Lecture 6,7 Editing file Objectives Color guide : Only in boys slides in Green Only in girls slides in Purple important in Red At the end of the lecture, students should be able to: Notes in Grey ● List the different parts of small intestine. ● Describe the anatomy of duodenum, jejunum & ileum regarding: (the shape, length, site of beginning & termination, peritoneal covering, arterial supply & lymphatic drainage) ● Differentiate between each part of duodenum regarding the length, level & relations. ● Differentiate between the jejunum & ileum regarding the characteristic anatomical features of each of them. ● List the different parts of large intestine. ● List the characteristic features of colon. ● Describe the anatomy of different parts of large intestine regarding: (the surface anatomy, peritoneal covering, relations, arterial & nerve supply) Small intestine The small intestine divided into : Fixed Part (No Mesentery): Free (Movable) Part (With Parts Duodenum* Mesentery): Jejunum & Ileum Shape C-shaped loop coiled tube Length 10 inches 6 meters (20 feet) Transverse Colon separates the Beginning At pyloro-duodenal junction at duodeno-jejunal flexure stomach/liver from the jejunum/ileum Termination At duodeno-jejunal flexure at ileo-ceacal flexure Peritoneal Covering Retroperitoneal mesentery of small intestine Divisions 4 parts --------- Foregut (above bile duct opening in 2nd part )& Midgut Embryological origin Midgut (below bile duct opening in 2nd part) So 2nd part has double -

Digestive System
Naziha Sultan Ahmed, BVMS, MSc Scientific degree (Assis. Prof.), Department of Anatomy College of Veterinary Medicine, University of Mosul, Mosul, Iraq https://orcid.org/0000-0002-2856-8277 https://www.researchgate.net/profile/Naziha_Ahmed Anatomy | Part 18| 2nd year 2019 Digestive System Fixation of the liver (Ligaments of the liver): 1-Lesser omentum: consist of two parts: a/Hepatogastric ligament: connect between hepatic porta and lesser curvature of the stomach . b/Hepatoduodenal ligament: connect between hepatic porta and the cranial part of the duodenum. 2-Coronary ligament: connect between the parietal (diaphragmatic ) surface of liver, with the diaphragm and the caudal vena cava. 3-Falciform ligament: connect between the notch of round ligament in the liver and the sternal part of the diaphragm. 4-Round ligament: the residue (remnants) of the umbilical vein of the fetus. 5-Hepatorenal ligament: connect between the right lobe of liver and the right kidney. 6-Right & left triangular ligaments: connect between the dorsal border of right and left lobes of the liver and the diaphragm . Both ligaments continue medially with the coronary ligament. CouAnatomy | Digestive system | Assis. Prof. Naziha Sultan Ahmed Page | 1 The pancreas: Pancreas has V-shape. It consists of base and two limbs (right & left limbs). *In horse: large pancreas body perforated by portal vein and long left limb, with short right limb (because of large size of cecum in horse ). The horse pancreas has two ducts: 1-Chief pancreatic duct: opens with bile duct at the major duodenal papilla. 2-Accessory pancreatic duct: opens at the minor duodenal papilla. *In dog: pancreas notched by the portal vein. -
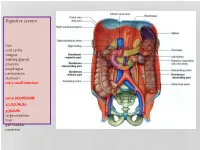
Digestive System
Digestive system Lips oral cavity tongue salivary glands pharynx esophagus peritoneum stomach m6-7 small intestine: cm25 DEODENUM: 2/5JEJUNUM: 3/5ILIUM: large intestine liver gall bladder pancreas :Duodenum Sup. Part Descending part Horizontal part Ascending part Major duodenal papilla Minor duodenal papilla : JEJUNUM / ILIUM From duodenojejunal curvature To Iliocecal Different jejunum & ilium 3Tenia coli: sigmoid 2 / rectum 0 Houstra / saccule Appendices epiploicae : No in Appendix / cecum / rectum Many in sigmoid DIFFERENT BETWEEN SMALL AND LARGE INTESTINE large intestine 1.5m Cecum appendix ascending colon transverse colon descending colon sigmoid colon rectum anal canal McBurneys point ascending colon transverse colon descending colon sigmoid colon :Rectum cm 12 Sacral flexure Puborectalis muscle Perineal flexure Rectum Ampulla : Anal canal cm 4 sup. = anal column / anal valve / anal sinus / pectinate line 2/3 Hiltons white line .inf 1/3 :Anal sphincter Internal sphincter External sphincter: deep / superficial / subcutaneous Liver: 1.5 kgr Located in Rt. & lf. Hypochondriac & epigastric region Surfaces: Sup. Anterior Surface: falciform ligament Inf. Surface: H shape fissure / porta hepatis / quadrate lobe / qudate lobe Post. Surface: bare area Rt. Surface: ribs 7-11 / Rt. lung Liver viewed from posterior Liver viewed from inferior Liver viewed from inferior Liver viewed from posterior :Liver vasculature 20%Hepatic artery 80%Portal vein Supra hepatic vein IVC :Nerve Sympathetic Parasympathetic From Vagus+ Phernic + celiac :Gall bladder Located in inf. Surface of liver cm7-10 Length: cm3-4 Wide: :Structure Fundus Body Infundibulum Neck Systic duct :Pancreas 2–L1 L cm15-20 Length: cm3Wide: cm2 Thickness: gram 90 :Structure Head :Uncinate process / sup. Mesenteric artery Neck: portal vein / sup. -

Carcinoid Tumor of the Minor Papilla in Complete Pancreas Divisum Presenting As Recurrent Abdominal Pain Yong Gil Kim, Tae Nyeun Kim*, Kyeong Ok Kim
Kim et al. BMC Gastroenterology 2010, 10:17 http://www.biomedcentral.com/1471-230X/10/17 CASE REPORT Open Access Carcinoid tumor of the minor papilla in complete pancreas divisum presenting as recurrent abdominal pain Yong Gil Kim, Tae Nyeun Kim*, Kyeong Ok Kim Abstract Background: Tumors of the minor papilla of the duodenum are extremely rare, and they are mostly neuroendocrine tumors, such as somatostatinomas and carcinoid tumors. However, true incidence of carcinoid tumors in minor papilla might be much higher, because patients with minor papillary tumors usually remain asymptomatic. We report a very unusual case of carcinoid tumor in a patient with complete pancreas divisum with a review of the literature. Case presentation: A 56-year-old female patient was referred for evaluation of pancreatic duct dilatation noted on abdominal ultrasonography and computerized tomography. She complained of intermittent epigastric pain for 6 months. A MRCP and ERCP revealed complete pancreas divisum with dilatation of the main pancreatic duct. On duodenoscopy, a small, yellows, subepithelial nodule was visualized at the minor papilla; biopsy of this lesion revealed a carcinoid tumor. She underwent a pylorus-preserving pancreaticoduodenectomy. The histologic evaluation showed a single nodule, 1 cm in diameter, in the submucosa with duodenal and vascular invasion and metastasis to the regional lymph nodes. Conclusion: Although the size of the carcinoid tumor was small and the tumor was hormonally inactive, the concomitant pancreas divisum led to an early diagnosis, the tumor had aggressive behavior. Carcinoid tumors of the minor papilla should be included in the differential diagnosis of recurrent abdominal pain or pancreatitis of unknown cause. -

The Gallbladder
The Gallbladder Anatomy of the gallbladder Location: Right cranial abdominal quadrant. In the gallbladder fossa of the liver. o Between the quadrate and right medial liver lobes. Macroscopic: Pear-shaped organ Fundus, body and neck. o Neck attaches, via a short cystic duct, to the common bile duct. Opens into the duodenum via sphincter of Oddi at the major duodenal papilla. Found on the mesenteric margin of orad duodenum. o 3-6 cm aboral to pylorus. 1-2cm of distal common bile duct runs intramural. Species differences: Dogs: o Common bile duct enters at major duodenal papilla. Adjacent to pancreatic duct (no confluence prior to entrance). o Accessory pancreatic duct enters at minor duodenal papilla. ± 2 cm aboral to major duodenal papilla. MAJOR conduit for pancreatic secretions. Cats: o Common bile duct and pancreatic duct converge before opening at major duodenal papilla. Thus, any surgical procedure that affects the major duodenal papilla can affect the exocrine pancreatic secretions in cats. o Accessory pancreatic duct only seen in 20% of cats. 1 Gallbladder wall: 5 histologically distinct layers. From innermost these include: o Epithelium, o Submucosa (consisting of the lamina propria and tunica submucosa), o Tunica muscularis externa, o Tunica serosa (outermost layer covers gallbladder facing away from the liver), o Tunica adventitia (outermost layer covers gallbladder facing towards the liver). Blood supply: Solely by the cystic artery (derived from the left branch of the hepatic artery). o Susceptible to ischaemic necrosis should its vascular supply become compromised. Function: Storage reservoir for bile o Concentrated (up to 10-fold), acidified (through epithelial acid secretions) and modified (by the addition of mucin and immunoglobulins) before being released into the gastrointestinal tract at the major duodenal. -

Aandp2ch25lecture.Pdf
Chapter 25 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • Most nutrients we eat cannot be used in existing form – Must be broken down into smaller components before body can make use of them • Digestive system—acts as a disassembly line – To break down nutrients into forms that can be used by the body – To absorb them so they can be distributed to the tissues • Gastroenterology—the study of the digestive tract and the diagnosis and treatment of its disorders 25-2 General Anatomy and Digestive Processes • Expected Learning Outcomes – List the functions and major physiological processes of the digestive system. – Distinguish between mechanical and chemical digestion. – Describe the basic chemical process underlying all chemical digestion, and name the major substrates and products of this process. 25-3 General Anatomy and Digestive Processes (Continued) – List the regions of the digestive tract and the accessory organs of the digestive system. – Identify the layers of the digestive tract and describe its relationship to the peritoneum. – Describe the general neural and chemical controls over digestive function. 25-4 Digestive Function • Digestive system—organ system that processes food, extracts nutrients, and eliminates residue • Five stages of digestion – Ingestion: selective intake of food – Digestion: mechanical and chemical breakdown of food into a form usable by -

Endoscopic Treatment of Pancreatic Diseases Via Duodenal Minor Papilla: 135 Cases Treated by Sphincterotomy, Endoscopic Pancreat
Annals of Clinical Gastroenterology and Hepatology Research Article More Information Submitted: 20 June 2019 Approved: 05 July 2019 Endoscopic treatment of pancreatic Published: 08 July 2019 How to cite this article: Tsuji T, Sun G, Sugiyama diseases via Duodenal Minor A, Amano Y, Mano S, et al. Endoscopic treatment of pancreatic diseases via Duodenal Minor Papilla: 135 cases treated by Sphincterotomy, Papilla: 135 cases treated by Endoscopic Pancreatic Duct Balloon Dilation (EPDBD), and Pancreatic Stenting (EPS). Ann Clin Gastroenterol Hepatol. 2019; 3: 012-019. Sphincterotomy, Endoscopic https://doi.org.10.29328/journal.acgh.1001009 Copyright: © 2019 Tsuji T, et al. This is an open Pancreatic Duct Balloon Dilation access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction (EPDBD), and Pancreatic Stenting in any medium, provided the original work is properly cited (EPS) Keywords: Minor Duodenal Papilla; Pancreatic Divisum; EPST; EPDBD-Endoscopic Pancreatic Tadao Tsuji1*, G Sun1, A Sugiyama1, Y Amano1, S Mano1, T Duct Balloon Dilation; EPS; Pancreatic Stone; Shinobi1, H Tanaka1, M Kubochi1, K Ohishi1, Y Moriya1, M Ono1, Pancreatic Pseudocyst T Masuda1, H Shinozaki2, H Kaneda2, H Katsura2, T Mizutani2, ISSN: 2640-2750 K Miura2, M Katoh2, K Yamafuji3, K Takeshima3, N Okamoto3, Y Hoshino4, N Tsurumi4, S Hisada4, J Won4, T Kogiso4, K Yatsuji4, M Iimura4, T Kakimoto5 and S Nyuhzuki6 1Saitama Cooperative Hospital, Gastroenterology, Japan 2Saitama City Hospital, Gastroenterology, -

Axis Scientific Human Digestive System (1/2 Size)
Axis Scientific Human Digestive System (1/2 Size) A-105865 48. Body of Pancreas 27. Transverse Colon 02. Hard Palate 47. Pancreatic Notch 07. Nasopharynx 05. Tooth 01. Lower Lip F. Large Intestine 06. Tongue 21. Jejunum A. Oral Cavity 09. Pharyngeal Tonsil 03. Soft Palate 08. Opening to Auditory Tube 28. E. Small Intestine 04. Uvula 11. Palatine Tonsil Descending Colon 40. Gallbladder 37. Round Ligament of Liver 22. Ileum 38. Quadrate Lobe 44. Proper Hepatic Artery 42. Common Hepatic Duct 45. Hepatic Portal Vein C. Esophagus 15. Fundus of Stomach 30. Rectum 29. Sigmoid Colon 13. Cardia 26. Ascending Colon 39. Caudate Lobe 24. Ileocecal Valve 35. Left Lobe of 16. Body of 34. Falciform Liver Stomach 41. Cystic Duct Ligament 36. Right Lobe of Liver G. Liver 31. Anal Canal 14. Pylorus D. Stomach 33. External 18. Duodenum Anal Sphincter Muscle 17. Pyloric Antrum 46. Head of Pancreas 23. Cecum 51. Accessory Pancreatic Duct 49. Tail of 50. Pancreatic 25. Vermiform 20. Minor Duodenal Papilla H. Pancreas Duct Pancreas Appendix 19. Major Duodenal Papilla 32. Internal Anal Sphincter Muscle 01. Lower Lip 20. Minor Duodenal Papilla 39. Caudate Lobe 02. Hard Palate 21. Jejunum 40. Gallbladder 03. Soft Palate 22. Ileum 41. Cystic Duct 42. Common Hepatic Duct 04. Uvula 23. Cecum 43. Common Bile Duct 05. Tooth 24. Ileocecal Valve 44. Proper Hepatic Artery 06. Tongue 25. Vermiform Appendix 45. Hepatic Portal Vein 07. Nasopharynx 26. Ascending Colon 46. Head of Pancreas 08. Opening to Auditory Tube 27. Transverse Colon 47. Pancreatic Notch 09. Pharyngeal Tonsil 28. -

Distance Between Major and Minor Duodenal Papilla from Pylorus – a Cadaveric Study M
International Journal of Anatomy and Research, Int J Anat Res 2018, Vol 6(2.2):5239-45. ISSN 2321-4287 Original Research Article DOI: https://dx.doi.org/10.16965/ijar.2018.167 DISTANCE BETWEEN MAJOR AND MINOR DUODENAL PAPILLA FROM PYLORUS – A CADAVERIC STUDY M. Pairoja Sultana *1, C.K. Lakshmidevi 2. *1Assistant professor in Anatomy, ACSR Govt. medical college, Nellore, Andhra Pradesh, India. 2 Professor and Head of the department of Anatomy, ACSR Govt. medical college, Nellore, Andhra Pradesh, India. ABSTRACT Introduction: Without the knowledge of the normal pattern of the duct system and its variations, a radiologist can’t interpret an Endoscopic Retrograde Cholangiopancreatography (ERCP) picture. So it becomes important to study the anatomy of pancreatic ducts, their relation to each other, to common bile duct and to duodenum in the available human cadavers. The present paper is about the study of distance between minor and major duodenal papilla from pylorus which was carried out on 96 cadaveric specimens of human duodeno-pancreas. To visualise and to see distance between minor and major duodenal papillae is necessary for the endoscopist who aims to perform the dilation, stenting, or papillotomy of the minor papilla. Materials and Methods: The study was conducted in 96 (64 male and 32 female) cadavers. Major and minor duodenal papillae were visualized through eosin dye installation in both common bile duct and the accessory pancreatic duct. The measurement of distance between the duodenal papillae and to pylorus was done in cm. Results: In the present work, the mean ± SD of the Distance between pylorus to MAP is 8.05 ± 1.71 cm, pylorus to MIP is 6.19 ± 1.49 cm, the major to minor duodenal papilla was on an average 2.02 ± 0.40 cm, these distances were more in males as compared to females. -

Small Intestines of the Horse
SMALL INTESTINES OF THE HORSE Duodenum: The cranial part of the duodenum is in contact with the visceral surface of the liver. It forms the sigmoid flexure and a dilatation (ampulla). The first curve of the sigmoid flexure is convex dorsally and the second curve is convex ventrally. The second curve of the sigmoid flexure is the cranial flexure where the body of the pancreas is attached. The major pancreatic duct and the bile duct open in this area at the major duodenal papilla inside the hepatopancreatic ampulla . The accessory pancreatic duct opens at the minor duodenal papilla opposite to the major duodenal papilla. The descending duodenum runs caudally between the visceral surface of the liver and the right dorsal colon. At the caudal pole of the right kidney it turns medially around the base of the cecum and the root of the mesentery to form the caudal flexure. The short ascending duodenum runs cranially and to the left. It is continued by the jejunum following the duodenojejunal flexure . Jejunum: Lies chiefly in the left dorsal part of the abdomen together with the small colon. It is attached to the dorsal abdominal wall by the long mesojejunum that allows great mobility of the bowel . This mobility may result in intestinal colic, due to volvulus, intussusception, or incarceration of the duodenal loops in the epiploic foramen or the vaginal ring. Ileum: Passes dorsally toward the lesser curvature of the base of the cecum, where it is partly telescoped into the the cecum so that the ileal orifice is surrounded by a fold of mucous membrane. -

Anatomical Variation in the Insertion of the Major Duodenal Papilla
Case report Anatomical variation in the insertion of the major duodenal papilla Franco, SSDR.*, Mendes, LFM., Fortes, LHS., Siqueira, SL. and Souza, IKF. Techniques and Experimental Surgery Laboratory, Department of Medical Sciences, Federal University of Ouro Preto – UFOP, Rua Paulo Magalhães Gomes, s/n, Bauxita, Campus Morro do Cruzeiro, CEP 35400-000, Ouro Preto, MG, Brazil *E-mail: [email protected] Abstract Introduction: The major duodenal papilla is usually located about eight centimeters distant from the pyloric ostium, in the descending portion of the duodenum. However, it is known that the distance of the papilla insertion may vary, and the ducts may not unite or not form a papillary structure. We describe the ectopic major duodenal papilla in two corpses owned by the Human Anatomy Laboratory at the Federal University of Ouro Preto. Materials and methods: Two corpses that had suffered prior formalinfixation were dissected and after the visualization of the anomalous character of the structures the distance from the pylorus was measured in each of them. In one of the corpses the major duodenal papilla was 5.2 cm distant from the pyloric ostium, lying posteromedial at the beginning of the upper duodenal flexure, while in the other one it was 6.7 cm distant from the pyloric ostium, lying posteromedial at the end of the upper duodenal flexure, between the first and second duodenal portions. A literature review of prevalence and surgical importance of this variation was performed through PubMed database and in anatomy and surgery textbooks. Results and conclusion: Dowdy and Disibeyaz described this type of variation and found 4% and 0,43% in their study, respectively.