Imperial-Ham-La:Layout 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report and Accounts 2019
IMPERIAL BRANDS PLC BRANDS IMPERIAL ANNUAL REPORT AND ACCOUNTS 2019 ACCOUNTS AND REPORT ANNUAL ANNUAL REPORT AND ACCOUNTS 2019 OUR PURPOSE WE CAN I OWN Our purpose is to create something Everything See it, seize it, is possible, make it happen better for the world’s smokers with together we win our portfolio of high quality next generation and tobacco products. In doing so we are transforming WE SURPRISE I AM our business and strengthening New thinking, My contribution new actions, counts, think free, our sustainability and value creation. exceed what’s speak free, act possible with integrity OUR VALUES Our values express who we are and WE ENJOY I ENGAGE capture the behaviours we expect Thrive on Listen, challenge, share, make from everyone who works for us. make it fun connections The following table constitutes our Non-Financial Information Statement in compliance with Sections 414CA and 414CB of the Companies Act 2006. The information listed is incorporated by cross-reference. Additional Non-Financial Information is also available on our website www.imperialbrands.com. Policies and standards which Information necessary to understand our business Page Reporting requirement govern our approach1 and its impact, policy due diligence and outcomes reference Environmental matters • Occupational health, safety and Environmental targets 21 environmental policy and framework • Sustainable tobacco programme International management systems 21 Climate and energy 21 Reducing waste 19 Sustainable tobacco supply 20 Supporting wood sustainability -

British American Tobacco's Submission to the WHO's
British American Tobacco’s submission to the WHO’s Framework Convention on Tobacco Control This is the submission of the British American Tobacco group of companies commenting on the WHO’s Framework Convention on Tobacco Control. We are the world’s most international tobacco group with an active presence in 180 countries. Our companies sell some of the world’s best known brands including Dunhill, Kent, State Express 555, Lucky Strike, Benson & Hedges, Rothmans and Pall Mall. Executive summary • The WHO’s proposed ‘Framework Convention on Tobacco Control’ is fundamentally flawed and will not achieve its objectives. • The tobacco industry, along with other industries involved in the manufacture and distribution of legal but risky products, is the subject of considerable public attention. It is important that the debate about tobacco remains open, objective, constructive and free from opportunistic criticism if we are effectively to address the real issues associated with tobacco. • British American Tobacco is responsible tobacco. We seek to operate in partnership with governments, who are significant stakeholders in our business, and other interested parties, based on our open acknowledgement that we make a risky product and therefore support sensible regulation. • British American Tobacco shares the World Health Organisation’s desire to reduce the health impact of tobacco use. This paper outlines British American Tobacco’s proposal for the sensible regulation of tobacco. • Our proposal will relieve the WHO of the cost and bureaucracy involved in its wish to become a single global tobacco regulator, leaving it free to do what it should be doing – policy orientation. Some facts about tobacco • Today over one billion adults, about one third of the world’s adult population, choose to smoke. -
FY 2020 Announcement.Pdf
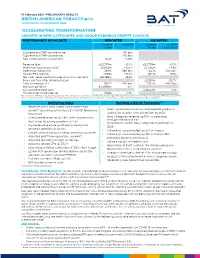
17 February 2021 –PRELIMINARY RESULTS BRITISH AMERICAN TOBACCO p.l.c. YEAR ENDED 31 DECEMBER 2020 ‘ACCELERATING TRANSFORMATION’ GROWTH IN NEW CATEGORIES AND GROUP EARNINGS DESPITE COVID-19 PERFORMANCE HIGHLIGHTS REPORTED ADJUSTED Current Vs 2019 Current Vs 2019 rates Rates (constant) Cigarette and THP volume share +30 bps Cigarette and THP value share +20 bps Non-Combustibles consumers1 13.5m +3.0m Revenue (£m) £25,776m -0.4% £25,776m +3.3% Profit from operations (£m) £9,962m +10.5% £11,365m +4.8% Operating margin (%) +38.6% +380 bps +44.1% +100 bps2 Diluted EPS (pence) 278.9p +12.0% 331.7p +5.5% Net cash generated from operating activities (£m) £9,786m +8.8% Free cash flow after dividends (£m) £2,550m +32.7% Cash conversion (%)2 98.2% -160 bps 103.0% +650 bps Borrowings3 (£m) £43,968m -3.1% Adjusted Net Debt (£m) £39,451m -5.3% Dividend per share (pence) 215.6p +2.5% The use of non-GAAP measures, including adjusting items and constant currencies, are further discussed on pages 48 to 53, with reconciliations from the most comparable IFRS measure provided. Note – 1. Internal estimate. 2. Movement in adjusted operating margin and operating cash conversion are provided at current rates. 3. Borrowings includes lease liabilities. Delivering today Building A Better TomorrowTM • Revenue, profit from operations and earnings • 1 growth* absorbing estimated 2.5% COVID-19 revenue 13.5m consumers of our non-combustible products , headwind adding 3m in 2020. On track to 50m by 2030 • New Categories revenue up 15%*, accelerating • Combustible revenue -

Companies Subject to Exclusion
Opdateret 01-08-2021 Vellivs Ekslusionsliste Velliv ønsker ikke at investere i selskaber, som bryder med Vellivs ESG-kriterier fastlagt i politikken for ansvarlig investering og aktivt ejerskab og som ikke udviser forandringsvilje i forhold til deres håndtering af ESG-risici. Endelig ønsker Velliv ikke at investere i tobaksselselskaber, selskaber med aktivteter i kontroversielle våben eller selskaber, hvor mere end 5 pct. af omsætningen stammer fra udvinding af kul og oliesand. Selsakb Kommentar 22nd Century Group, Inc. Tobacco AECOM Contr.Weapons Aerojet Rocketdyne Holdings, Inc. Contr.Weapons Aerojet Rocketdyne, Inc. Contr.Weapons Aeroteh SA Contr.Weapons Airbus SE Contr.Weapons Al-Eqbal Co. for Investment Plc Tobacco ALLETE, Inc. Kul Alliance Holdings GP LP Coal Alliance Resource Operating Partners LP Coal Alliance Resource Partners LP Coal Alpha Metallurgical Resources, Inc Coal Alpha Natural Resources Inc Coal Altadis Emisiones Financieras SA Tobacco Altadis SA Tobacco AltaGas Ltd. Gas/ESG Altius Minerals Corporation Coal Altria Group, Inc. Tobacco Anglo American plc Kul Anglo Pacific Group plc Coal Arch Resources, Inc. Coal Arcis Resources Corp. Tobacco Aryt Industries Ltd. Contr.Weapons Asenovgrad Tabac AD Tobacco Ashtrom Group Ltd. Normviolation>human rights Athabasca Oil Corporation Oilsand Avco Corp. Contr.Weapons B.A.T. Capital Corp. Tobacco B.A.T. Finance BV Tobacco B.A.T. International Finance Plc Tobacco Babcock International Group plc Contr.Weapons BADECO ADRIA dd Tobacco BAE Systems (Finance) Ltd. Contr.Weapons BAE Systems plc Contr.Weapons Bat Brasil Tobacco Bathurst Resources Limited Coal Baytex Energy (LP) Ltd. Oilsand Baytex Energy Corp. Oilsand Bellatora, Inc. Tobacco BHP Group Limited Kul BHP Group Plc Kul BlackPearl Resources, Inc. -

Annual Report 2013
Annual Report 2013 “ Being active and having a positive outlook on life is what keeps me going every day.” Overview of 2013 “ Our performance in 2013 was defined by remarkable &R D output and further delivery of sustained financial performance for our shareholders.” Please go to page 4 for more More at gsk.com Performance highlights £26.5bn £8.0bn £7.0bn £5.2bn Group turnover Core* operating profit Total operating profit Returned to shareholders 6 112.2p 112.5p 13% Major medicines approved Core* earnings per share Total earnings per share Estimated return on R&D investment 10 6 1st 1st Potential phase III study starts in 2014/15 Potential medicines with phase III data in Access to Medicines Index Pharmaceutical company to sign AllTrials expected 2014/15 campaign for research transparency Front cover story Betty, aged 65, (pictured) has Chronic “ Health is important to me, Obstructive Pulmonary Disease (COPD). She only has 25% lung capacity. This means I try to take care of my she finds even everyday tasks difficult, but medicines and inhaled oxygen allow her to health with all the tools live as normal a life as she can. Betty’s mindset I have and do the best is to stay busy and active, so every week she goes to rehab exercise classes. that I can with it.” COPD is a disease of the lungs that leads to Betty, COPD patient, damaged airways, causing them to become North Carolina, USA narrower and making it harder for air to get in and out. 210 million people around the world are estimated to have COPD. -

BAT INTERNATIONAL FINANCE Plc
BASE PROSPECTUS B.A.T. INTERNATIONAL FINANCE p.l.c. (incorporated with limited liability in England and Wales) BRITISH AMERICAN TOBACCO HOLDINGS (THE NETHERLANDS) B.V. (incorporated with limited liability in The Netherlands) B.A.T. NETHERLANDS FINANCE B.V. (incorporated with limited liability in The Netherlands) B.A.T CAPITAL CORPORATION (incorporated with limited liability in the State of Delaware, United States of America) £25,000,000,000 Euro Medium Term Note Programme unconditionally and irrevocably guaranteed by BRITISH AMERICAN TOBACCO p.l.c. (incorporated with limited liability in England and Wales) and each of the Issuers (except where it is the relevant Issuer) On 6 July 1998, each of B.A.T. International Finance p.l.c. (“BATIF”), B.A.T Capital Corporation (“BATCAP”) and B.A.T Finance B.V. (“BATFIN”) entered into a Euro Medium Term Note Programme (the “Programme”) for the issue of Euro Medium Term Notes (the “Notes”). On 16 April 2003, British American Tobacco Holdings (The Netherlands) B.V. (“BATHTN”) acceded to the Programme as an issuer and, where relevant, a guarantor and BATFIN was removed as an issuer and a guarantor under the Programme. On 9 December 2011, BATCAP was removed as an issuer and a guarantor under the Programme. On 16 May 2014, B.A.T. Netherlands Finance B.V. (“BATNF”) acceded to the Programme as an issuer and, where relevant, a guarantor. On 31 May 2017, BATCAP acceded to the Programme as an issuer and, where relevant, a guarantor. BATIF, BATHTN, BATNF and BATCAP are each, in their capacities as issuers under the Programme, an “Issuer” and together referred to as the “Issuers”. -

On Appeal from the Court of Appeal for British Columbia
Court File No. 33563 IN THE SUPREME COURT OF CANADA (ON APPEAL FROM THE COURT OF APPEAL FOR BRITISH COLUMBIA) BETWEEN: THE ATTORNEY GENERAL OF CANADA APPELLANT/RESPONDENT BY CROSS-APPEAL (THIRD PARTY) – and – IMPERIAL TOBACCO CANADA LIMITED, ROTHMANS, BENSON & HEDGES INC., ROTHMANS INC., JTI-MACDONALD CORP., B.A.T INDUSTRIES P.L.C., BRITISH AMERICAN TOBACCO (INVESTMENTS) LIMITED, CARRERAS ROTHMANS LIMITED, PHILIP MORRIS USA INC., PHILIP MORRIS INTERNATIONAL INC., R.J. REYNOLDS TOBACCO COMPANY and R.J. REYNOLDS TOBACCO INTERNATIONAL, INC. RESPONDENTS/APPELLANTS BY CROSS-APPEAL (APPELLANTS) – and – HER MAJESTY THE QUEEN IN RIGHT OF BRITISH COLUMBIA RESPONDENT (RESPONDENT) – and – ATTORNEY GENERAL OF BRITISH COLUMBIA and ATTORNEY GENERAL OF NEW BRUNSWICK INTERVENERS CONSOLIDATED FACTUM OF RESPONDENTS ON APPEAL AND CONSOLIDATED FACTUM OF APPELLANTS ON CROSS-APPEAL OF ROTHMANS, BENSON & HEDGES INC., ROTHMANS INC., PHILIP MORRIS USA INC. AND PHILIP MORRIS INTERNATIONAL INC. (pursuant to Rules 42 and 43 of the Rules of the Supreme Court of Canada) COUNSEL FOR ROTHMANS, BENSON & OTTAWA AGENT FOR ROTHMANS, HEDGES INC. AND ROTHMANS INC. BENSON & HEDGES INC. AND MACAULAY McCOLL LLP ROTHMANS INC. 1575 – 650 West Georgia Street GOWLING LAFLEUR HENDERSON LLP Vancouver, British Columbia V6B 4N9 2600 – 160 Elgin Street Telephone: 604-687-9811 Ottawa, Ontario K1P 1C3 Facsimile: 604-687-8716 Telephone: 613-233-1781 KENNETH N. AFFLECK, Q.C. Facsimile: 613-563-9869 HENRY S. BROWN, Q.C. COUNSEL FOR PHILIP MORRIS USA OTTAWA AGENT FOR PHILIP MORRIS INC. USA INC. DAVIS & COMPANY LLP GOWLING LAFLEUR HENDERSON LLP 2800 – 666 Burrard Street 2600 – 160 Elgin Street Vancouver, British Columbia V7Y 1K2 Ottawa, Ontario K1P 1C3 Telephone: 604-687-9444 Telephone: 613-233-1781 Facsimile: 604-687-1612 Facsimile: 613-563-9869 D. -

International Marketing Principles
British American Tobacco’s International Marketing Principles At British American Tobacco we believe in upholding high standards of corporate behaviour. We agree that the tobacco industry should be regulated, but we also think we should be able to communicate in a responsible way with adult tobacco consumers about our products, in order to grow market share. British American Tobacco’s International The Marketing Principles apply to the Marketing Principles provide a consistent marketing of all British American and responsible approach to marketing Tobacco’s combustible tobacco across the Group. They replace the products. International Marketing Standards which were launched in 2001 and updated in 2007. The Marketing Principles are our minimum The new Marketing Principles ensure our standard and will be applied even when they approach reflects developments in are stricter than local laws. However, if local marketing, technology and changing laws or other voluntary codes in markets are regulations and stakeholder expectations. stricter than or override our Marketing Principles, then we will abide by those laws The Marketing Principles comprise four core or voluntary codes. principles which we believe are at the heart of responsible tobacco marketing. The We will monitor and audit our performance rationale for each principle is explained and against the Marketing Principles and report illustrated by a set of core standards which our findings in the Group’s Sustainability show how they should be applied in our Report. The appendix at the end of this communications with consumers. document contains more information about the governance of the Marketing Principles. The Marketing Principles outline what all our Group Companies must consider when We expect all Group companies and anyone planning their tobacco marketing activities working on our behalf to adopt these but they do not offer standards for every Marketing Principles and seek to apply them activity. -

Annex 1: Parker Review Survey Results As at 2 November 2020
Annex 1: Parker Review survey results as at 2 November 2020 The data included in this table is a representation of the survey results as at 2 November 2020, which were self-declared by the FTSE 100 companies. As at March 2021, a further seven FTSE 100 companies have appointed directors from a minority ethnic group, effective in the early months of this year. These companies have been identified through an * in the table below. 3 3 4 4 2 2 Company Company 1 1 (source: BoardEx) Met Not Met Did Not Submit Data Respond Not Did Met Not Met Did Not Submit Data Respond Not Did 1 Admiral Group PLC a 27 Hargreaves Lansdown PLC a 2 Anglo American PLC a 28 Hikma Pharmaceuticals PLC a 3 Antofagasta PLC a 29 HSBC Holdings PLC a InterContinental Hotels 30 a 4 AstraZeneca PLC a Group PLC 5 Avast PLC a 31 Intermediate Capital Group PLC a 6 Aveva PLC a 32 Intertek Group PLC a 7 B&M European Value Retail S.A. a 33 J Sainsbury PLC a 8 Barclays PLC a 34 Johnson Matthey PLC a 9 Barratt Developments PLC a 35 Kingfisher PLC a 10 Berkeley Group Holdings PLC a 36 Legal & General Group PLC a 11 BHP Group PLC a 37 Lloyds Banking Group PLC a 12 BP PLC a 38 Melrose Industries PLC a 13 British American Tobacco PLC a 39 Mondi PLC a 14 British Land Company PLC a 40 National Grid PLC a 15 BT Group PLC a 41 NatWest Group PLC a 16 Bunzl PLC a 42 Ocado Group PLC a 17 Burberry Group PLC a 43 Pearson PLC a 18 Coca-Cola HBC AG a 44 Pennon Group PLC a 19 Compass Group PLC a 45 Phoenix Group Holdings PLC a 20 Diageo PLC a 46 Polymetal International PLC a 21 Experian PLC a 47 -

Imperial Tobacco Canada Limited and Imperial Tobacco Company Limited
Court File No. CV-19-616077-00CL Imperial Tobacco Canada Limited and Imperial Tobacco Company Limited PRE-FILING REPORT OF THE PROPOSED MONITOR March 12, 2019 TABLE OF CONTENTS GENERAL .....................................................................................................................................1 INTRODUCTION ............................................................................................................................1 BACKGROUND ..............................................................................................................................6 FORUM .........................................................................................................................................14 ACCOMMODATION AGREEMENT ................................................................................................15 THE TOBACCO CLAIMANT REPRESENTATIVE .............................................................................17 IMPERIAL’S CASH FLOW FORECAST ..........................................................................................18 COURT-ORDERED CHARGES .......................................................................................................20 CHAPTER 15 PROCEEDINGS ........................................................................................................23 RELIEF SOUGHT ...........................................................................................................................24 CONCLUSION ...............................................................................................................................25 -

A FOCUS on HARM REDUCTION Why It
A FOCUS ON HARM REDUCTION Why it mattersSUSTAINABILITY FOCUS REPORT 2013: How we address the public health impact of our products A nicotine molecule In this report Focusing on the facts 2 At the core of our business strategy 3 Innovative nicotine products 4 ‘Safer’ tobacco products: the research 7 So, what’s next? 9 Our Chief Executive on why it matters b British American Tobacco Harm reduction: sustainability focus report 2013 Because it’s crucial to the future of our business. Surely tobacco harm reduction should just be about What areas are you concentrating on? getting people to quit smoking? Our approach to harm reduction has two distinct areas: The only way to be certain of avoiding the serious health risks nicotine-based alternatives and reduced-risk tobacco products. associated with smoking is not to smoke at all. However, despite In the nicotine category, we have established a stand-alone increasingly strict tobacco control policies, many people continue business solely dedicated to this area. This brings together our to smoke. And the World Health Organisation estimates that existing Nicoventures business with CN Creative, the e-cigarette 1 many more will do so in the future . So realistically the ‘quit or die’ company we acquired at the end of last year, into a single business approach to reducing the public health impact of smoking simply which will continue to operate under the Nicoventures name. isn’t enough. This business has already launched its first e-cigarette in the UK, For adults that choose to continue to smoke, tobacco harm which will be expanded into further markets in the coming year. -

HARM REDUCTION the Opportunity
HARM REDUCTION The opportunity Sustainability Focus Report 2014 CLICK TO READ 1 HARM REDUCTION A NEW WAY 8 FORWARD? 2 STEP INSIDE: WHAT IS HARM VIDEO TOUR OF REDUCTION? OUR R&D LABS HARM WHAT THE TRANSPARENCY INDEPENDENT AND REDUCTION EVIDENCE 7 WORLD-CLASS 3 TELLS US SCIENCE The opportunity TO OPEN ADDITIONAL OUR PREFERRED INFORMATION, click on APPROACH TO NEW PRODUCTS any blue plus sign. REGULATING SAFER CHOICES? NICOTINE PRODUCTS A PRAGMATIC PUBLIC HEALTH 4 6 POLICY INDEPENDENT ASSURANCE TO CLOSE ADDITIONAL INFORMATION, click on GET IN the yellow cross sign. 5 TOUCH HARM REDUCTION? A NEW WAY FORWARD OUR CHIEF EXECUTIVE This is an exciting time to be leading British American Tobacco. We have a new Group vision to satisfy consumer moments in tobacco and beyond, and an enhanced strategy with sustainability as one of its key pillars. This reflects our commitment to building shared value for our shareholders and for society. Harm reduction is crucial to achieving this vision: offering less risky tobacco and nicotine products helps to meet consumer demand, and so makes genuine commercial sense, while also being the right thing to do. This is not new to BAT – harm reduction has been a strategic focus for us for a long time. But it’s only relatively recently that we’ve seen the emergence of a whole new generation of alternative products – with both the rise of e-cigarettes and the potential for innovative tobacco heating devices. We think these products can have the greatest consumer appeal and, therefore, could significantly help in reducing smoking-related disease.