Glucose and Insulin Treatment of Insulinoma Cells Results In
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Single-Cell RNA-Sequencing-Based Crispri Screening Resolves Molecular Drivers of Early Human Endoderm Development
University of Massachusetts Medical School eScholarship@UMMS Open Access Articles Open Access Publications by UMMS Authors 2019-04-16 Single-Cell RNA-Sequencing-Based CRISPRi Screening Resolves Molecular Drivers of Early Human Endoderm Development Ryan M. Genga University of Massachusetts Medical School Et al. Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/oapubs Part of the Amino Acids, Peptides, and Proteins Commons, Cell Biology Commons, Cells Commons, Developmental Biology Commons, Embryonic Structures Commons, Genetic Phenomena Commons, and the Nucleic Acids, Nucleotides, and Nucleosides Commons Repository Citation Genga RM, Kernfeld EM, Parsi KM, Parsons TJ, Ziller MJ, Maehr R. (2019). Single-Cell RNA-Sequencing- Based CRISPRi Screening Resolves Molecular Drivers of Early Human Endoderm Development. Open Access Articles. https://doi.org/10.1016/j.celrep.2019.03.076. Retrieved from https://escholarship.umassmed.edu/oapubs/3818 Creative Commons License This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 4.0 License. This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in Open Access Articles by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. Report Single-Cell RNA-Sequencing-Based CRISPRi Screening Resolves Molecular Drivers of Early Human Endoderm Development Graphical Abstract Authors Ryan M.J. Genga, Eric M. Kernfeld, Krishna M. Parsi, Teagan J. Parsons, Michael J. Ziller, Rene´ Maehr Correspondence [email protected] In Brief Genga et al. utilize a single-cell RNA- sequencing-based CRISPR interference approach to screen transcription factors predicted to have a role in human definitive endoderm differentiation. -

Single Cell Transcriptomics Reveal Temporal Dynamics of Critical Regulators of Germ Cell Fate During Mouse Sex Determination
bioRxiv preprint doi: https://doi.org/10.1101/747279; this version posted November 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Single cell transcriptomics reveal temporal dynamics of critical regulators of germ 2 cell fate during mouse sex determination 3 Authors: Chloé Mayère1,2, Yasmine Neirijnck1,3, Pauline Sararols1, Chris M Rands1, 4 Isabelle Stévant1,2, Françoise Kühne1, Anne-Amandine Chassot3, Marie-Christine 5 Chaboissier3, Emmanouil T. Dermitzakis1,2, Serge Nef1,2,*. 6 Affiliations: 7 1Department of Genetic Medicine and Development, University of Geneva, 1211 Geneva, 8 Switzerland; 9 2iGE3, Institute of Genetics and Genomics of Geneva, University of Geneva, 1211 10 Geneva, Switzerland; 11 3Université Côte d'Azur, CNRS, Inserm, iBV, France; 12 Lead Contact: 13 *Corresponding Author: Serge Nef, 1 rue Michel-Servet CH-1211 Genève 4, 14 [email protected]. + 41 (0)22 379 51 93 15 Running Title: Single cell transcriptomics of germ cells 1 bioRxiv preprint doi: https://doi.org/10.1101/747279; this version posted November 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 16 Abbreviations; 17 AGC: Adrenal Germ Cell 18 GC: Germ cell 19 OGC: Ovarian Germ Cell 20 TGC: Testicular Germ Cell 21 scRNA-seq: Single-cell RNA-Sequencing 22 DEG: Differentially Expressed Gene 23 24 25 Keywords: 26 Single-cell RNA-Sequencing (scRNA-seq), sex determination, ovary, testis, gonocytes, 27 oocytes, prospermatogonia, meiosis, gene regulatory network, germ cells, development, 28 RNA splicing 29 2 bioRxiv preprint doi: https://doi.org/10.1101/747279; this version posted November 2, 2020. -

Watsonjn2018.Pdf (1.780Mb)
UNIVERSITY OF CENTRAL OKLAHOMA Edmond, Oklahoma Department of Biology Investigating Differential Gene Expression in vivo of Cardiac Birth Defects in an Avian Model of Maternal Phenylketonuria A THESIS SUBMITTED TO THE GRADUATE FACULTY In partial fulfillment of the requirements For the degree of MASTER OF SCIENCE IN BIOLOGY By Jamie N. Watson Edmond, OK June 5, 2018 J. Watson/Dr. Nikki Seagraves ii J. Watson/Dr. Nikki Seagraves Acknowledgements It is difficult to articulate the amount of gratitude I have for the support and encouragement I have received throughout my master’s thesis. Many people have added value and support to my life during this time. I am thankful for the education, experience, and friendships I have gained at the University of Central Oklahoma. First, I would like to thank Dr. Nikki Seagraves for her mentorship and friendship. I lucked out when I met her. I have enjoyed working on this project and I am very thankful for her support. I would like thank Thomas Crane for his support and patience throughout my master’s degree. I would like to thank Dr. Shannon Conley for her continued mentorship and support. I would like to thank Liz Bullen and Dr. Eric Howard for their training and help on this project. I would like to thank Kristy Meyer for her friendship and help throughout graduate school. I would like to thank my committee members Dr. Robert Brennan and Dr. Lilian Chooback for their advisement on this project. Also, I would like to thank the biology faculty and staff. I would like to thank the Seagraves lab members: Jailene Canales, Kayley Pate, Mckayla Muse, Grace Thetford, Kody Harvey, Jordan Guffey, and Kayle Patatanian for their hard work and support. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -
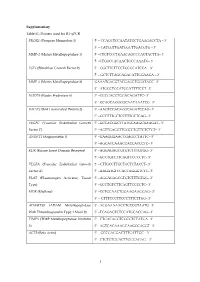
1 Supplementary Table S1. Primers Used for RT-Qpcr PROX1
Supplementary Table S1. Primers used for RT-qPCR PROX1 (Prospero Homeobox 1) 5’ – CCAGCTCCAATATGCTGAAGACCTA – 3’ 5’ – CATCGTTGATGGCTTGACGTG – 3‘ MMP-1 (Matrix Metallopeptidase 1) 5' –CTGTCCCTGAACAGCCCAGTACTTA– 3' 5' –CTGGCCACAACTGCCAAATG– 3' FGF2 (Fibroblast Growth Factor 2) 5′ - GGCTTCTTCCTGCGCATCCA – 3′ 5′ – GCTCTTAGCAGACATTGGAAGA – 3′ MMP-3 (Matrix Metallopeptidase 3) GAAATGAGGTACGAGCTGGATACC– 3’ 5’ –ATGGCTGCATCGATTTTCCT– 3’ NUDT6 (Nudix Hydrolase 6) 5’ –GGCGAGCTGGACAGATTC– 3’ 5’ –GCAGCAGGGGCAATAAATCG– 3’ BAIAP2 (BAI1 Associated Protein 2) 5’ –AAGTCCACAGGCAGATCCAG– 3’ 5’ –GCCTTTGCTCCTTTGCTCAG– 3’ VEGFC (Vascular Endothelial Growth 5’ –GCCACGGCTTATGCAAGCAAAGAT– 3’ Factor C) 5’ –AGTTGAGGTTGGCCTGTTCTCTGT– 3’ ANGPT1 (Angiopoietin 1) 5’ –GAAGGGAACCGAGCCTATTC– 3’ 5’ –AGCATCAAACCACCATCCTC– 3’ KDR (Kinase Insert Domain Receptor) 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ VEGFA (Vascular Endothelial Growth 5’ –CTTGCCTTGCTGCTCTACCT– 3’ Factor A) 5’ –AAGATGTCCACCAGGGTCTC– 3’ PLAT (Plasminogen Activator, Tissue 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ Type) 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ MDK (Midkine) 5’ –CCTGCAACTGGAAGAAGGAG– 3’ 5’ -- CTTTCCCTTCCCTTTCTTGG– 3’ ADAMTS9 (ADAM Metallopeptidase 5’ –ACGAAAAACCTGCCGTAATG– 3’ With Thrombospondin Type 1 Motif 9) 5’ –TCAGAGTCTCCATGCACCAG– 3’ TIMP3 (TIMP Metallopeptidase Inhibitor 5’ –CTGACAGGTCGCGTCTATGA– 3’ 3) 5’ –AGTCACAAAGCAAGGCAGGT– 3’ ACTB (Beta Actin) 5’ – GCCGAGGACTTTGATTGC – 3’ 5’– CTGTGTGGACTTGGGAGAG – 3’ 1 Figure S1. Efficient silencing of PROX1 in CGTH-W-1 and FTC-133 cells. Western blotting analysis shows a decrease in PROX1 protein level by targeting with siRNAs purchased from Santa Cruz (SC) and Sigma-Aldrich (SA) in both CGTH-W-1 and FTC-133 cell line. Beta-actin was used as a loading control of protein lysates. Figure S2. The tube formation assay. The silencing of PROX1 in CGTH-W-1 and FTC-133 cells enhances the angiogenesis in vitro of endothelial cells. HUVECs were cultured in 96-well plates coated with a semi-solid Matrigel. -

Supplemental Materials ZNF281 Enhances Cardiac Reprogramming
Supplemental Materials ZNF281 enhances cardiac reprogramming by modulating cardiac and inflammatory gene expression Huanyu Zhou, Maria Gabriela Morales, Hisayuki Hashimoto, Matthew E. Dickson, Kunhua Song, Wenduo Ye, Min S. Kim, Hanspeter Niederstrasser, Zhaoning Wang, Beibei Chen, Bruce A. Posner, Rhonda Bassel-Duby and Eric N. Olson Supplemental Table 1; related to Figure 1. Supplemental Table 2; related to Figure 1. Supplemental Table 3; related to the “quantitative mRNA measurement” in Materials and Methods section. Supplemental Table 4; related to the “ChIP-seq, gene ontology and pathway analysis” and “RNA-seq” and gene ontology analysis” in Materials and Methods section. Supplemental Figure S1; related to Figure 1. Supplemental Figure S2; related to Figure 2. Supplemental Figure S3; related to Figure 3. Supplemental Figure S4; related to Figure 4. Supplemental Figure S5; related to Figure 6. Supplemental Table S1. Genes included in human retroviral ORF cDNA library. Gene Gene Gene Gene Gene Gene Gene Gene Symbol Symbol Symbol Symbol Symbol Symbol Symbol Symbol AATF BMP8A CEBPE CTNNB1 ESR2 GDF3 HOXA5 IL17D ADIPOQ BRPF1 CEBPG CUX1 ESRRA GDF6 HOXA6 IL17F ADNP BRPF3 CERS1 CX3CL1 ETS1 GIN1 HOXA7 IL18 AEBP1 BUD31 CERS2 CXCL10 ETS2 GLIS3 HOXB1 IL19 AFF4 C17ORF77 CERS4 CXCL11 ETV3 GMEB1 HOXB13 IL1A AHR C1QTNF4 CFL2 CXCL12 ETV7 GPBP1 HOXB5 IL1B AIMP1 C21ORF66 CHIA CXCL13 FAM3B GPER HOXB6 IL1F3 ALS2CR8 CBFA2T2 CIR1 CXCL14 FAM3D GPI HOXB7 IL1F5 ALX1 CBFA2T3 CITED1 CXCL16 FASLG GREM1 HOXB9 IL1F6 ARGFX CBFB CITED2 CXCL3 FBLN1 GREM2 HOXC4 IL1F7 -

Subunit Gene Expression Through Smad-Binding and Homeobox Elements
0888-8809/05/$15.00/0 Molecular Endocrinology 19(10):2610–2623 Printed in U.S.A. Copyright © 2005 by The Endocrine Society doi: 10.1210/me.2005-0047 Activin Regulates Luteinizing Hormone -Subunit Gene Expression through Smad-Binding and Homeobox Elements Djurdjica Coss, Varykina G. Thackray, Chu-Xia Deng, and Pamela L. Mellon Departments of Reproductive Medicine and Neuroscience (D.C., V.G.T., P.L.M.), Center for Reproductive Science and Medicine, University of California, San Diego, La Jolla, California 92093- 0674; and Genetics of Development and Disease Branch (C.-X.D.), National Institute of Diabetes and Downloaded from https://academic.oup.com/mend/article/19/10/2610/2738010 by guest on 23 September 2021 Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland 20892 LH -subunit (LH), which is essential for ovulation site found in this region of the promoter. Juxta- and reproductive fitness, is synthesized specifi- posed to the HB are three Smad-binding elements cally in pituitary gonadotropes. In this study, we (SBEs), which are essential for LH induction. In- show that LH gene expression is induced by ac- terestingly, two of the SBEs are also critical for tivin in mouse primary pituitary cells if the cells are basal expression of the LH gene. We demonstrate treated within 24 h after dispersion in culture. Fur- that Smad proteins are necessary and sufficient for thermore, male mice deficient in Smad3, and there- activin induction of the LH gene. Furthermore, fore in activin signaling, have lower expression of Smad proteins can bind one of the identified SBEs. -

Physical and Linkage Mapping of Mammary-Derived Expressed Sequence Tags in Cattle
Genomics 83 (2004) 148–152 www.elsevier.com/locate/ygeno Physical and linkage mapping of mammary-derived expressed sequence tags in cattle E.E. Connor,a,* T.S. Sonstegard,a J.W. Keele,b G.L. Bennett,b J.L. Williams,c R. Papworth,c C.P. Van Tassell,a and M.S. Ashwella a U.S. Beltsville Agricultural Research Center, ARS, U.S. Department of Agriculture, 10300 Baltimore Avenue, Beltsville, MD 20705, USA b U.S. Meat Animal Research Center, ARS, U.S. Department of Agriculture, P.O. Box 166, Clay Center, NE 68933-0166, USA c Roslin Institute (Edinburgh), Roslin, Midlothian EH25 9PS, Scotland, United Kingdom Received 2 June 2003; accepted 5 July 2003 Abstract This study describes the physical and linkage mapping of 42 gene-associated markers developed from mammary gland-derived expressed sequence tags to the cattle genome. Of the markers, 25 were placed on the USDA reference linkage map and 37 were positioned on the Roslin 3000-rad radiation hybrid (RH) map, with 20 assignments shared between the maps. Although no novel regions of conserved synteny between the cattle and the human genomes were identified, the coverage was extended for bovine chromosomes 3, 7, 15, and 29 compared with previously published comparative maps between human and bovine genomes. Overall, these data improve the resolution of the human–bovine comparative maps and will assist future efforts to integrate bovine RH and linkage map data. Crown Copyright D 2003 Published by Elsevier Inc. All rights reserved. Keywords: RH mapping; Linkage mapping; SNP; Cattle; EST Selection of positional candidate genes controlling eco- pig [4,5], and cattle [6], and serve as a resource for nomically important traits in cattle requires a detailed candidate gene identification. -

Supplemental Information
Supplemental information Dissection of the genomic structure of the miR-183/96/182 gene. Previously, we showed that the miR-183/96/182 cluster is an intergenic miRNA cluster, located in a ~60-kb interval between the genes encoding nuclear respiratory factor-1 (Nrf1) and ubiquitin-conjugating enzyme E2H (Ube2h) on mouse chr6qA3.3 (1). To start to uncover the genomic structure of the miR- 183/96/182 gene, we first studied genomic features around miR-183/96/182 in the UCSC genome browser (http://genome.UCSC.edu/), and identified two CpG islands 3.4-6.5 kb 5’ of pre-miR-183, the most 5’ miRNA of the cluster (Fig. 1A; Fig. S1 and Seq. S1). A cDNA clone, AK044220, located at 3.2-4.6 kb 5’ to pre-miR-183, encompasses the second CpG island (Fig. 1A; Fig. S1). We hypothesized that this cDNA clone was derived from 5’ exon(s) of the primary transcript of the miR-183/96/182 gene, as CpG islands are often associated with promoters (2). Supporting this hypothesis, multiple expressed sequences detected by gene-trap clones, including clone D016D06 (3, 4), were co-localized with the cDNA clone AK044220 (Fig. 1A; Fig. S1). Clone D016D06, deposited by the German GeneTrap Consortium (GGTC) (http://tikus.gsf.de) (3, 4), was derived from insertion of a retroviral construct, rFlpROSAβgeo in 129S2 ES cells (Fig. 1A and C). The rFlpROSAβgeo construct carries a promoterless reporter gene, the β−geo cassette - an in-frame fusion of the β-galactosidase and neomycin resistance (Neor) gene (5), with a splicing acceptor (SA) immediately upstream, and a polyA signal downstream of the β−geo cassette (Fig. -

Single Cell Profiling of CRISPR/Cas9-Induced OTX2 Deficient Retinas Reveals Fate Switch from Restricted Progenitors
bioRxiv preprint doi: https://doi.org/10.1101/538710; this version posted February 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Single cell profiling of CRISPR/Cas9-induced OTX2 deficient retinas reveals fate switch from restricted progenitors Miruna G. Ghinia Tegla1, Diego F. Buenaventura1, 2, Diana Y. Kim1, Cassandra Thakurdin1, Kevin C. Gonzalez1, 3, Mark M. Emerson1,2* 1 Department of Biology, The City College of New York, City University of New York, New York, NY, 10031; United States of America 2 Biology Ph.D. Program, Graduate Center, City University of New York, New York, NY, 10031; United States of America 3 Present address: Doctoral Program in Neurobiology and Behavior, Columbia University, New York, NY 10032; United States of America *Corresponding author: [email protected] Email addresses: Miruna G. Ghinia Tegla: [email protected] Diego F. Buenaventura: [email protected] Diana Y. Kim: [email protected] Cassandra Thakurdin: [email protected] Kevin C. Gonzalez: [email protected] Mark M. Emerson: [email protected] Running title: Single cell analysis of retinal cell fate changes induced by OTX2 mutagenesis bioRxiv preprint doi: https://doi.org/10.1101/538710; this version posted February 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Development of the vertebrate eye, like many developmental systems, depends on genes that are used iteratively in multiple distinct processes. -

The Id-Protein Family in Developmental and Cancer-Associated Pathways Cornelia Roschger and Chiara Cabrele*
Roschger and Cabrele Cell Communication and Signaling (2017) 15:7 DOI 10.1186/s12964-016-0161-y REVIEW Open Access The Id-protein family in developmental and cancer-associated pathways Cornelia Roschger and Chiara Cabrele* Abstract Inhibitors of DNA binding and cell differentiation (Id) proteins are members of the large family of the helix-loop- helix (HLH) transcription factors, but they lack any DNA-binding motif. During development, the Id proteins play a key role in the regulation of cell-cycle progression and cell differentiation by modulating different cell-cycle regulators both by direct and indirect mechanisms. Several Id-protein interacting partners have been identified thus far, which belong to structurally and functionally unrelated families, including, among others, the class I and II bHLH transcription factors, the retinoblastoma protein and related pocket proteins, the paired-box transcription factors, and the S5a subunit of the 26 S proteasome. Although the HLH domain of the Id proteins is involved in most of their protein-protein interaction events, additional motifs located in their N-terminal and C-terminal regions are required for the recognition of diverse protein partners. The ability of the Id proteins to interact with structurally different proteins is likely to arise from their conformational flexibility: indeed, these proteins contain intrinsically disordered regions that, in the case of the HLH region, undergo folding upon self- or heteroassociation. Besides their crucial role for cell-fate determination and cell-cycle progression during development, other important cellular events have been related to the Id-protein expression in a number of pathologies. Dysregulated Id-protein expression has been associated with tumor growth, vascularization, invasiveness, metastasis, chemoresistance and stemness, as well as with various developmental defects and diseases. -

Mutation Analysis of Genes Within the Dynactin Complex in a Cohort of Hereditary Peripheral Neuropathies
Clin Genet 2016: 90: 127–133 © 2015 John Wiley & Sons A/S. Printed in Singapore. All rights reserved Published by John Wiley & Sons Ltd CLINICAL GENETICS doi: 10.1111/cge.12712 Original Article Mutation analysis of genes within the dynactin complex in a cohort of hereditary peripheral neuropathies a a Tey S., Ahmad-Annuar A., Drew A.P., Shahrizaila N., Nicholson G.A., S. Tey , A. Ahmad-Annuar , Kennerson M.L. Mutation analysis of genes within the dynactin complex in A.P. Drewb, N. Shahrizailac, , a cohort of hereditary peripheral neuropathies. G.A. Nicholsonb d and Clin Genet 2016: 90: 127–133. © John Wiley & Sons A/S. Published by M.L. Kennersonb,d John Wiley & Sons Ltd, 2015 aDepartment of Biomedical Science, The cytoplasmic dynein–dynactin genes are attractive candidates for Faculty of Medicine, University of Malaya, b neurodegenerative disorders given their functional role in retrograde Kuala Lumpur, Malaysia, Northcott transport along neurons. The cytoplasmic dynein heavy chain (DYNC1H1) Neuroscience Laboratory, ANZAC Research Institute, and Sydney Medical gene has been implicated in various neurodegenerative disorders, and School, University of Sydney, Sydney, dynactin 1 (DCTN1) genes have been implicated in a wide spectrum of Australia, cDepartment of Medicine, disorders including motor neuron disease, Parkinson’s disease, spinobulbar Faculty of Medicine, University of Malaya, muscular atrophy and hereditary spastic paraplegia. However, the Kuala Lumpur, Malaysia, and dMolecular involvement of other dynactin genes with inherited peripheral neuropathies Medicine Laboratory, Concord Hospital, (IPN) namely, hereditary sensory neuropathy, hereditary motor neuropathy Sydney, Australia and Charcot–Marie–Tooth disease is under reported. We screened eight genes; DCTN1-6 and ACTR1A and ACTR1B in 136 IPN patients using Key words: Charcot–Marie–Tooth – whole-exome sequencing and high-resolution melt (HRM) analysis.