Modelling 3D Craniofacial Growth Trajectories for Population Comparison and Classification Illustrated Using Sex-Differences
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Hands-On Human Evolution: a Laboratory Based Approach
Hands-on Human Evolution: A Laboratory Based Approach Developed by Margarita Hernandez Center for Precollegiate Education and Training Author: Margarita Hernandez Curriculum Team: Julie Bokor, Sven Engling A huge thank you to….. Contents: 4. Author’s note 5. Introduction 6. Tips about the curriculum 8. Lesson Summaries 9. Lesson Sequencing Guide 10. Vocabulary 11. Next Generation Sunshine State Standards- Science 12. Background information 13. Lessons 122. Resources 123. Content Assessment 129. Content Area Expert Evaluation 131. Teacher Feedback Form 134. Student Feedback Form Lesson 1: Hominid Evolution Lab 19. Lesson 1 . Student Lab Pages . Student Lab Key . Human Evolution Phylogeny . Lab Station Numbers . Skeletal Pictures Lesson 2: Chromosomal Comparison Lab 48. Lesson 2 . Student Activity Pages . Student Lab Key Lesson 3: Naledi Jigsaw 77. Lesson 3 Author’s note Introduction Page The validity and importance of the theory of biological evolution runs strong throughout the topic of biology. Evolution serves as a foundation to many biological concepts by tying together the different tenants of biology, like ecology, anatomy, genetics, zoology, and taxonomy. It is for this reason that evolution plays a prominent role in the state and national standards and deserves thorough coverage in a classroom. A prime example of evolution can be seen in our own ancestral history, and this unit provides students with an excellent opportunity to consider the multiple lines of evidence that support hominid evolution. By allowing students the chance to uncover the supporting evidence for evolution themselves, they discover the ways the theory of evolution is supported by multiple sources. It is our hope that the opportunity to handle our ancestors’ bone casts and examine real molecular data, in an inquiry based environment, will pique the interest of students, ultimately leading them to conclude that the evidence they have gathered thoroughly supports the theory of evolution. -

Is the Skeleton Male Or Female? the Pelvis Tells the Story
Activity: Is the Skeleton Male or Female? The pelvis tells the story. Distinct features adapted for childbearing distinguish adult females from males. Other bones and the skull also have features that can indicate sex, though less reliably. In young children, these sex-related features are less obvious and more difficult to interpret. Subtle sex differences are detectable in younger skeletons, but they become more defined following puberty and sexual maturation. What are the differences? Compare the two illustrations below in Figure 1. Female Pelvic Bones Male Pelvic Bones Broader sciatic notch Narrower sciatic notch Raised auricular surface Flat auricular surface Figure 1. Female and male pelvic bones. (Source: Smithsonian Institution, illustrated by Diana Marques) Figure 2. Pelvic bone of the skeleton in the cellar. (Source: Smithsonian Institution) Skull (Cranium and Mandible) Male Skulls Generally larger than female Larger projections behind the Larger brow ridges, with sloping, ears (mastoid processes) less rounded forehead Square chin with a more vertical Greater definition of muscle (acute) angle of the jaw attachment areas on the back of the head Figure 3. Male skulls. (Source: Smithsonian Institution, illustrated by Diana Marques) Female Skulls Smoother bone surfaces where Smaller projections behind the muscles attach ears (mastoid processes) Less pronounced brow ridges, Chin more pointed, with a larger, with more vertical forehead obtuse angle of the jaw Sharp upper margins of the eye orbits Figure 4. Female skulls. (Source: Smithsonian Institution, illustrated by Diana Marques) What Do You Think? Comparing the skull from the cellar in Figure 5 (below) with the illustrated male and female skulls in Figures 3 and 4, write Male or Female to note the sex depicted by each feature. -

A Description of the Geological Context, Discrete Traits, and Linear Morphometrics of the Middle Pleistocene Hominin from Dali, Shaanxi Province, China
AMERICAN JOURNAL OF PHYSICAL ANTHROPOLOGY 150:141–157 (2013) A Description of the Geological Context, Discrete Traits, and Linear Morphometrics of the Middle Pleistocene Hominin from Dali, Shaanxi Province, China Xinzhi Wu1 and Sheela Athreya2* 1Laboratory for Human Evolution, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing 100044, China 2Department of Anthropology, Texas A&M University, College Station, TX 77843 KEY WORDS Homo heidelbergensis; Homo erectus; Asia ABSTRACT In 1978, a nearly complete hominin Afro/European Middle Pleistocene Homo and align it fossil cranium was recovered from loess deposits at the with Asian H. erectus.Atthesametime,itdisplaysa site of Dali in Shaanxi Province, northwestern China. more derived morphology of the supraorbital torus and It was subsequently briefly described in both English supratoral sulcus and a thinner tympanic plate than and Chinese publications. Here we present a compre- H. erectus, a relatively long upper (lambda-inion) occi- hensive univariate and nonmetric description of the pital plane with a clear separation of inion and opis- specimen and provide comparisons with key Middle thocranion, and an absolute and relative increase in Pleistocene Homo erectus and non-erectus hominins brain size, all of which align it with African and Euro- from Eurasia and Africa. In both respects we find pean Middle Pleistocene Homo. Finally, traits such as affinities with Chinese H. erectus as well as African the form of the frontal keel and the relatively short, and European Middle Pleistocene hominins typically broad midface align Dali specifically with other referred to as Homo heidelbergensis.Specifically,the Chinese specimens from the Middle Pleistocene Dali specimen possesses a low cranial height, relatively and Late Pleistocene, including H. -

Download Poster
Sutural Variability in the Hominoid Anterior Cranial Fossa Robert McCarthy, Monica Kunkel, Department of Biological Sciences, Benedictine University Email contact information: [email protected]; [email protected] SAMPLE ABSTRACT Table 2. Results split by age. Table 4. Specimens used in scaling analyses. Group Age A-M434; M555 This study Combined In anthropoids, the orbital plates of frontal bone meet at a “retro-ethmoid” Species Symbol FMNH CMNH USNM % Contact S-E S-E S-E % % % frontal suture in the midline anterior cranial fossa (ACF), separating the contact/n contact/n contact/n presphenoid and mesethmoid bones. Previous research indicates that this Hylobatid 0 0 23 13.0 Hylobatid Adult 0/6 0 4/13 30.8 4/19 21.1 configuration appears variably in chimpanzees and gorillas and infrequently in modern humans, with speculation that its incidence is related to differential Juvenile 0/2 0 3/10 30.0 3/12 25.0 Orangutan 6 0 37 100.0 growth of the brain and orbits, size of the brow ridges or face, or upper facial Combined 0/9 0 7/23 30.4 7/32 21.9 prognathism. We collected qualitative and quantitative data from 164 Gorilla 3 0 4 28.6 previously-opened cranial specimens from 15 hominoid species in addition to A Orangutan Adult 12/12 100.0 21/21 100.0 21/21 100.0 Chimpanzee 1 0 7 57.1 qualitative observations on non-hominoid and hominin specimens in order to Juvenile 13/13 100.0 13/13 100.0 13/13 100.0 (1) document sutural variability in the primate ACF, (2) rethink the evolutionary trajectory of frontal bone contribution to the midline ACF, and Combined 26/26 100.0 34/34 100.0 34/34 34/34 Human 0 83 0 91.8 (3) create a database of ACF observations and measurements which can be Gorilla Adult 3/7 42.9 1/2 50.0 3/7 42.9 used to test hypotheses about structural relationships in the hominoid ACF. -
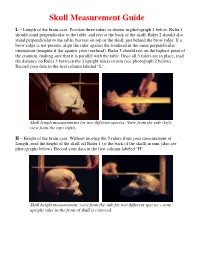
AP Skull Measurement Guide, Laminate
Skull Measurement Guide L – Length of the brain case: Position three rulers as shown in photograph 1 below. Ruler 1 should stand perpendicular to the table, and rest at the back of the skull. Ruler 2 should also stand perpendicular to the table, but rest on top of the skull, just behind the brow ridge. If a brow ridge is not present, align the ruler against the forehead in the same perpendicular orientation (imagine it flat against your forehead). Ruler 3 should rest on the highest point of the cranium, making sure that it is parallel with the table. Once all 3 rulers are in place, read the distance on Ruler 3 between the 2 upright rulers in mm (see photograph 2 below). Record your data in the first column labeled “L”. Skull length measurements for two different species: View from the side (left), view from the top (right). H – Height of the brain case: Without moving the 3 rulers from your measurement of Length, read the height of the skull off Ruler 1 (at the back of the skull) in mm (also see photographs below). Record your data in the first column labeled “H”. Skull height measurement: view from the side for two different species – note: upright ruler in the front of skull is removed. Skull Measurement Guide W – Width of the brain case: Position 3 rulers as shown in the photographs below. Rulers 1 and 2 should stand perpendicular to the table and flat against the skull (imagine 2 rulers flat against where the ears would be positioned). -

Artifacts and Commingled Skeletal Remains from a Well on the Medical College of Virginia Campus: Appendices Douglas W
Virginia Commonwealth University VCU Scholars Compass Office of the President Documents VCU University Archives 6-18-2012 Artifacts and Commingled Skeletal Remains from a Well on the Medical College of Virginia Campus: Appendices Douglas W. Owsley Smithsonian Institution Karin Bruwelheide Smithsonian Institution Merry Outlaw Archaeological and Cultural Solutions Richard L. Jantz University of Tennessee-Knoxville Follow this and additional works at: http://scholarscompass.vcu.edu/arch001 Downloaded from http://scholarscompass.vcu.edu/arch001/5 This Research Report is brought to you for free and open access by the VCU University Archives at VCU Scholars Compass. It has been accepted for inclusion in Office of the President Documents by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. Appendix I. Artifact Catalog C o Site Art Manf Form u Ware Material Color Remarks ID Id Tech n t Flat, everted lip, rolled in; pinched in neck; cylindrical body; slightly 44HE Mouth 001 Jar 28 Glass Colorless domed base with rough 814 Blown pontil on exterior; base dia.: 5 3/4"; 28 fragments Translucent white fabric; glossy white glaze; interior underglaze 44HE Ch Blue and Wheel- 002 Saucer 1 Porcelain cobalt blue decoration 814 Porcelain White Thrown consisting of hanging dots above diaper motif; ca. 1785-1820 Translucent white fabric; colorless glaze; interior hand painted overglaze 44HE Ch Wheel- decoration consisting of 003 Bowl 1 Porcelain Polychrome 814 Porcelain Thrown horizontal lines of black -

The Frontal Bone As a Proxy for Sex Estimation in Humans: a Geometric
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2014 The frontal bone as a proxy for sex estimation in humans: a geometric morphometric analysis Lucy Ann Edwards Hochstein Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Social and Behavioral Sciences Commons Recommended Citation Hochstein, Lucy Ann Edwards, "The frontal bone as a proxy for sex estimation in humans: a geometric morphometric analysis" (2014). LSU Master's Theses. 1749. https://digitalcommons.lsu.edu/gradschool_theses/1749 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. THE FRONTAL BONE AS A PROXY FOR SEX ESTIMATION IN HUMANS: A GEOMETRIC MORPHOMETRIC ANALYSIS A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Anthropology in The Department of Geography and Anthropology By Lucy A. E. Hochstein B.A., George Mason University, 2009 May 2014 ACKNOWLEDGEMENTS Completing a master’s thesis was the most terrifying aspect of graduate school and I must acknowledge the people and pets that helped me on this adventure. I could not have asked for a better committee chair than Dr. Ginny Listi, who stuck by me when everything fell apart and was always willing to offer support and help me find solutions. -

Eyebrow Peak Position Redefined © 2010 the American Society for Aesthetic Plastic Surgery, Inc
Facial Surgery Aesthetic Surgery Journal 30(3) 297 –300 Eyebrow Peak Position Redefined © 2010 The American Society for Aesthetic Plastic Surgery, Inc. Reprints and permission: http://www .sagepub.com/ journalsPermissions.nav DOI: 10.1177/1090820X10369918 Sylvia Pham; Bradon Wilhelmi, MD; and Arian Mowlavi, MD www.aestheticsurgeryjournal.com Abstract Background: The aesthetically appealing eyebrow shape has been defined by its arch, located near the junction between the medial two-thirds and lateral one-third. The position of this arch has been historically described by arbitrary anatomical landmarks that have no logical structural relationship. Moreover, selection of endoscopic brow lift incision sites that define vector of pull and fixation points have been variably described. Objectives: The authors examine the position of the deep temporal fusion line to determine whether it can act as a more accurate and functional landmark than prior anatomical landmarks for the eyebrow peak position. Methods: Eyebrows were measured in 50 subjects from the medial aspect of the eyebrow to the a) deep temporal fusion line (ridge), b) eyebrow peak (arch), c) lateral aspect of the brow, and d) lateral limbus. Pearson's correlation, descriptive statistics, and student's t test results were obtained. Results: Eyebrow measurements demonstrated that the deep temporal fusion line is the most precise indicator of brow peak position among all examined landmarks. The Pearson correlation value was strongest between brow peak and deep temporal fusion line (P = .860) and a t test confirmed this observation with no significant difference between brow peak and deep temporal fusion line. The lateral limbus and medial two-thirds lateral one- third junction more accurately predict brow peak in females, but the deep temporal fusion line is an equally reliable predictor of brow peak for males and females. -

Windsor Mound by Karin Anderson and Michael Lindeen Edited by Karin Anderson August 30, 1990 Archaeological Resources Management
windsor Mound by Karin Anderson and Michael Lindeen edited by Karin Anderson August 30, 1990 Archaeological Resources Management Service Ball state University -- Acknowledgements Thanks to Mike Lindeen for providing me access to his data. Thanks to Dr. Glenn of Ball state University for help with the measurements and advice. Thanks to Don Cochran for his patience and guidance. Special thanks to Jeff McCoy without whose technical assistance this would have been handwritten. Thanks to Dallas Evans and Amy Johnson for their expertise. i - Table of contents Introduction - Windsor Mound 1 Historical Background 2 Methodology 3 cranial Descriptions 5 Summary of Cranial Descriptions 16 Conclusion 17 References Cited 19 Appendix A - Blank Data Form 21 Appendix B - Cranial Measurements 27 Cranial Indices 29 Cranial Observations 32 Table 1. Sex and Age 36 Appendix C - Photographs 38 Appendix D - Ceramic Analysis 45 Illustration 46 Appendix E - Faunal Analysis 48 Appendix F - Lithic Analysis 50 ii Windsor Mound (12-R-1) is presently located in a small wooded area, surrounded by agricultural lands. It is one of the largest mounds in east-central Indiana, fifteen feet high and over 100 feet across the base (Cochran field notes on file, ARMS). Approximately one-third of this impressive and important mound remains unexcavated, to date. This report is an attempt to extract information from the Windsor materials on loan to Ball State University's Archaeological Laboratory. To this end, the human skeletal material was inventoried and measurements, observations and photographs were taken. The ceramics were inventoried, identified and illustrated where necessary. The faunal material was identified where that was possible. -

Archaic Human Remains from Hualongdong, China, and Middle Pleistocene Human Continuity and Variation
Archaic human remains from Hualongdong, China, and Middle Pleistocene human continuity and variation Xiu-Jie Wua,b, Shu-Wen Peia,b,1, Yan-Jun Caic,d,e,1, Hao-Wen Tonga,b, Qiang Lia,b, Zhe Donga,b,f,g, Jin-Chao Shengh, Ze-Tian Jinh, Dong-Dong Maa,b,f, Song Xinga,b, Xiao-Li Lii, Xing Chengc,f, Hai Chenge,j, Ignacio de la Torrek, R. Lawrence Edwardsj, Xi-Cheng Gongg, Zhi-Sheng Anc,d, Erik Trinkausl,1, and Wu Liua,b,1 aKey Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, 100044 Beijing, China; bCAS Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, 100044 Beijing, China; cState Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, Chinese Academy of Sciences, 710061 Xi’an, China; dCAS Center for Excellence in Quaternary Science and Global Change, Chinese Academy of Sciences, 710061 Xi’an, China; eInstitute of Global Environmental Change, Xi’an Jiaotong University, 710049 Xi’an, China; fUniversity of Chinese Academy of Sciences, 100049 Beijing, China; gAnhui Provincial Institute of Cultural Relics and Archaeology, 230061 Hefei, China; hOffice for Cultural Relics Administration of Dongzhi County, 247200 Dongzhi, China; iBeijing Museum of Natural History, 100050 Beijing, China; jDepartment of Earth Sciences, University of Minnesota, Minneapolis, MN 55455; kInstitute of Archaeology, University College London, WC1H 0PY London, United Kingdom; and lDepartment of Anthropology, Washington University, St. Louis, MO 63130 Contributed by Erik Trinkaus, March 25, 2019 (sent for review February 12, 2019; reviewed by Sheela Athreya and Christopher J. -

Skull Anatomy
Skull Anatomy Cranial Capacity (braincase size) – cranium minus the face; may or may not indicate the level of intelligence (Intelligence is determined by overall size of the organism, where it lives, and the level of brain development.) Post-orbital Construction – the narrowing of the braincase behind the eye orbits; the orbit is the eye socket Sagittal Crest – ridge on top of the cranium for attachment of chewing muscles; runs down the middle of the top of the head; seen in predators that chase and bring down prey Forehead – frontal bone (A vertical forehead indicates an expanded frontal lobe, which is used for planning, reasoning, judgement, future consequences from current actions.) Foramen Magnum – the opening in the base of the skull where the spinal cord enters (The location indicates whether an organism is bipedal or quadruped.) Brow Ridge – bony ridge over the eye orbit (This reinforces weaker bones of the face due to strong chewing muscles.) Facial Prognathism – forward projection of the jaws and teeth beyond the cheek bone (A less forward projection indicates a reduction in tooth size due to a change in diet and cooked food. Chin angle relates to this.) Chin – slopes backward without a point or forward with a pointed end Dental Arcade – shape of the teeth row when viewed from above (U-shaped or parabolic- shaped) Teeth – Incisors – for cutting Canines – for tearing Molars – for grinding Canine Diastema – gap between the upper incisors and canines, and lower canines and premolars (the diastema is either present or absent to accommodate the canine from the opposite jaw) . -
Patterns of Morphological Integration in Modern Human Crania: Evaluating Hypotheses of Modularity Using Geometric Morphometrics
PATTERNS OF MORPHOLOGICAL INTEGRATION IN MODERN HUMAN CRANIA: EVALUATING HYPOTHESES OF MODULARITY USING GEOMETRIC MORPHOMETRICS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Adam Kolatorowicz Graduate Program in Anthropology The Ohio State University 2015 Dissertation Committee: Jeffrey K. McKee, Advisor Paul W. Sciulli Samuel D. Stout Mark Hubbe Copyrighted by Adam Kolatorowicz 2015 ABSTRACT This project examines patterns of phenotypic integration in modern human cranial morphology using geometric morphometric methods. It is theoretically based in the functional paradigm of craniofacial growth and morphological integration. The hypotheses being addressed are: 1) cranial form is influenced by secular trends, sex, and phylogenetic history of the population and 2) integration patterns wherein the basicranium is the keystone feature best explains the relationships among in cranial modules. Geometric morphometric methods were used to collect and analyze three- dimensional coordinate data of 152 endocranial and ectocranial landmarks from 391 anatomically modern human crania. These crania are derived from temporally historic and recent groups in the United States spanning both sexes and across several ancestral groups. Landmark data were subjected to generalized Procrustes analysis and then areas of shape variation were identified via principal components analysis of shape coordinates. Discriminant function analysis and canonical variate analysis identified regions that can be used to separate groups. Temporal period, ancestry, and sex all have significant effects on mean shape. Age-at-death accounts for a small proportion of the total variation. Modern individuals have higher, narrower vaults with highly arched palates ii and historic individuals have short, wider vaults with shallower palates.