The Approach to the Patient with an Unknown Overdose Timothy B
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

EH&S COVID-19 Chemical Disinfectant Safety Information
COVID-19 CHEMICAL DISINFECTANT SAFETY INFORMATION Updated June 24, 2020 The COVID-19 pandemic has caused an increase in the number of disinfection products used throughout UW departments. This document provides general information about EPA-registered disinfectants, such as potential health hazards and personal protective equipment recommendations, for the commonly used disinfectants at the UW. Chemical Disinfectant Base / Category Products Potential Hazards Controls ● Ethyl alcohol Highly flammable and could form explosive Disposable nitrile gloves Alcohols ● ● vapor/air mixtures. ● Use in well-ventilated areas away from o Clorox 4 in One Disinfecting Spray Ready-to-Use ● May react violently with strong oxidants. ignition sources ● Alcohols may de-fat the skin and cause ● Wear long sleeve shirt and pants ● Isopropyl alcohol dermatitis. ● Closed toe shoes o Isopropyl Alcohol Antiseptic ● Inhalation of concentrated alcohol vapor 75% Topical Solution, MM may cause irritation of the respiratory tract (Ready to Use) and effects on the central nervous system. o Opti-Cide Surface Wipes o Powell PII Disinfectant Wipes o Super Sani Cloth Germicidal Wipe 201 Hall Health Center, Box 354400, Seattle, WA 98195-4400 206.543.7262 ᅵ fax 206.543.3351ᅵ www.ehs.washington.edu ● Formaldehyde Formaldehyde in gas form is extremely Disposable nitrile gloves for Aldehydes ● ● flammable. It forms explosive mixtures with concentrations 10% or less ● Paraformaldehyde air. ● Medium or heavyweight nitrile, neoprene, ● Glutaraldehyde ● It should only be used in well-ventilated natural rubber, or PVC gloves for ● Ortho-phthalaldehyde (OPA) areas. concentrated solutions ● The chemicals are irritating, toxic to humans ● Protective clothing to minimize skin upon contact or inhalation of high contact concentrations. -

Carbamate Pesticides Aldicarb Aldicarb Sulfoxide Aldicarb Sulfone
Connecticut General Statutes Sec 19a-29a requires the Commissioner of Public Health to annually publish a list setting forth all analytes and matrices for which certification for testing is required. Connecticut ELCP Drinking Water Analytes Revised 05/31/2018 Microbiology Total Coliforms Fecal Coliforms/ E. Coli Carbamate Pesticides Legionella Aldicarb Cryptosporidium Aldicarb Sulfoxide Giardia Aldicarb Sulfone Carbaryl Physicals Carbofuran Turbidity 3-Hydroxycarbofuran pH Methomyl Conductivity Oxamyl (Vydate) Minerals Chlorinated Herbicides Alkalinity, as CaCO3 2,4-D Bromide Dalapon Chloride Dicamba Chlorine, free residual Dinoseb Chlorine, total residual Endothall Fluoride Picloram Hardness, Calcium as Pentachlorophenol CaCO3 Hardness, Total as CaCO3 Silica Chlorinated Pesticides/PCB's Sulfate Aldrin Chlordane (Technical) Nutrients Dieldrin Endrin Ammonia Heptachlor Nitrate Heptachlor Epoxide Nitrite Lindane (gamma-BHC) o-Phosphate Metolachlor Total Phosphorus Methoxychlor PCB's (individual aroclors) Note 1 PCB's (as decachlorobiphenyl) Note 1 Demands Toxaphene TOC Nitrogen-Phosphorus Compounds Alachlor Metals Atrazine Aluminum Butachlor Antimony Diquat Arsenic Glyphosate Barium Metribuzin Beryllium Paraquat Boron Propachlor Cadmium Simazine Calcium Chromium Copper SVOC's Iron Benzo(a)pyrene Lead bis-(2-ethylhexyl)phthalate Magnesium bis-(ethylhexyl)adipate Manganese Hexachlorobenzene Mercury Hexachlorocyclopentadiene Molybdenum Nickel Potassium Miscellaneous Organics Selenium Dibromochloropropane (DBCP) Silver Ethylene Dibromide (EDB) -

Methamphetamine Presentations to an Emergency Department: Management and Complications
Isoardi Katherine (Orcid ID: 0000-0002-1176-7923) Abstract Objective: There is little recent published data characterising methamphetamine intoxication. This study aims to describe the clinical effects, management, complications and disposition of patients with methamphetamine exposure. Methods: This is a retrospective review of patients presenting with methamphetamine intoxication to an Emergency Department in 2016. All presentations were extracted from a relational database and each medical record reviewed. Demographics, clinical features, complications and disposition were extracted. Results: There were 378 presentations of 329 patients (234 males [71%]), median age 31 years (range 16-68 years). The commonest clinical effect was acute behavioural disturbance, occurring in 295 (78%) presentations. This was successfully managed with oral sedation alone in 180 (61%) patients with the remainder receiving parenteral sedation. Other effects included tachycardia in 212 (56%), hypertension in 160 (42%) and hyperthermia in 17 (4%) presentations. No antihypertensives were given. One patient was actively cooled. Complications included 21 (30%) presentations with rhabdomyolysis and 43 (11%) presentations with acute kidney injury. There were two seizures, three intracranial bleeds and one myocardial infarction. The majority of This is the author manuscript accepted for publication and has undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/1742-6723.13219 This article is protected by copyright. All rights reserved. patients (310 [82%]) were managed solely within the emergency department. The median length of stay was 14 hours. There were 41(11%) mental health admissions. -
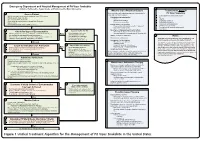
Snake Bite Protocol
Lavonas et al. BMC Emergency Medicine 2011, 11:2 Page 4 of 15 http://www.biomedcentral.com/1471-227X/11/2 and other Rocky Mountain Poison and Drug Center treatment of patients bitten by coral snakes (family Ela- staff. The antivenom manufacturer provided funding pidae), nor by snakes that are not indigenous to the US. support. Sponsor representatives were not present dur- At the time this algorithm was developed, the only ing the webinar or panel discussions. Sponsor represen- antivenom commercially available for the treatment of tatives reviewed the final manuscript before publication pit viper envenomation in the US is Crotalidae Polyva- ® for the sole purpose of identifying proprietary informa- lent Immune Fab (ovine) (CroFab , Protherics, Nash- tion. No modifications of the manuscript were requested ville, TN). All treatment recommendations and dosing by the manufacturer. apply to this antivenom. This algorithm does not con- sider treatment with whole IgG antivenom (Antivenin Results (Crotalidae) Polyvalent, equine origin (Wyeth-Ayerst, Final unified treatment algorithm Marietta, Pennsylvania, USA)), because production of The unified treatment algorithm is shown in Figure 1. that antivenom has been discontinued and all extant The final version was endorsed unanimously. Specific lots have expired. This antivenom also does not consider considerations endorsed by the panelists are as follows: treatment with other antivenom products under devel- opment. Because the panel members are all hospital- Role of the unified treatment algorithm -

Lindane Shampoo Reference Number: CP.PMN.09 Effective Date: 11.01.06 Last Review Date: 08.20 Line of Business: Medicaid Revision Log
Clinical Policy: Lindane Shampoo Reference Number: CP.PMN.09 Effective Date: 11.01.06 Last Review Date: 08.20 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Lindane is an ectoparasiticide and ovicide effective against Pediculus humanus capitis (head lice), Pthirus pubis (crab lice), and their ova. FDA Approved Indication(s) Lindane Shampoo is indicated for the treatment of head lice (infestations of Pediculus humanus capitis), crab lice (infestations of Pthirus pubis), and their ova only in patients who cannot tolerate other approved therapies, or have failed treatment with other approved therapies. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. It is the policy of health plans affiliated with Centene Corporation® that Lindane shampoo is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Head Lice (must meet all): 1. Diagnosis of Pediculus capitis (head lice); 2. Failure of two preferred agents indicated for head lice (see Appendix B for examples), at least one of which was used within the past 60 days, unless ALL preferred agents for head lice are contraindicated or clinically significant adverse effects are experienced; 3. Request does not exceed 1 bottle (60 mL) per treatment course. Approval duration: 14 days B. Crab Lice (must meet all): 1. Diagnosis of Phthirus pubis (crab lice); 2. Failure of pyrethrins/piperonyl butoxide AND permethrin 1% cream, used in last 60 days, unless both are contraindicated or clinically significant adverse effects are experienced; 3. -

Activated Charcoal for Acute Poisoning: One Toxicologist's Journey
J. Med. Toxicol. (2010) 6:190–198 DOI 10.1007/s13181-010-0046-1 REVIEW ARTICLE Activated Charcoal for Acute Poisoning: One Toxicologist’s Journey Kent R. Olson Published online: 20 May 2010 # The Author(s) 2010. This article is published with open access at Springerlink.com Keywords Activated charcoal . Gastrointestinal As summarized by Matthew [13], Harstad and Danish decontamination . Poisoning . Drug overdose colleagues reported in 1942 that relatively little phenobar- bital was recovered by lavage even shortly after overdose [14] and this, along with their finding of particles of When I began studying clinical toxicology in 1981, the issue charcoal in the lungs of patients who died, led them to call of gastrointestinal decontamination after acute ingestion for the abandonment of gastric lavage for poisoning.) “ ” seemed pretty well settled: universal antidote, apomor- In recent years, my colleagues and I have continued to – phine and salt wateremesiswerenolongerused[1, 2]; and I debate the value of various methods of gastric decontam- was taught that barring a specific contraindication, the awake ination and the role and risks of activated charcoal, and it is patient was given syrup of ipecac to induce emesis, and the clear to me that the issue remains muddy. Some have taken drowsy or uncooperative patient was lavaged [3]. After a firm stand that no treatment should be recommended that is gastric emptying, everyone received activated charcoal not supported by evidence from a randomized controlled trial (AC). The only controversy seemed to be over whether one (RCT). Position statements published jointly by the Amer- should add a cathartic to speed gastrointestinal transit [4]. -

Unintentional Fentanyl Overdoses Among Persons Who Thought They
Morbidity and Mortality Weekly Report Notes from the Field Unintentional Fentanyl Overdoses Among emergency departments; 2) holding a multiagency press Persons Who Thought They Were Snorting conference; 3) conducting media interviews; 4) informing Cocaine — Fresno, California, January 7, 2019 law enforcement, prehospital providers, and the public about Patil Armenian, MD1; Jeffrey D. Whitman, MD2; Adina Badea, PhD2; naloxone distribution and use; 5) educating persons on Whitney Johnson, MD1; Chelsea Drake, MS1; Simranjit Singh Dhillon3; the proper disposal of old or new but unused medications Michelle Rivera3; Nicklaus Brandehoff, MD1; Kara L. Lynch, PhD2 through the Fresno County Department of Behavioral Health/ California Health Collaborative drop-off containers§; and On January 7, 2019, three patients arrived at the Community 6) publicizing the California Central Valley Opioid Safety Regional Medical Center emergency department in Fresno, Coalition webpage,¶ which provides information about California, after snorting (i.e., nasally insufflating) white pow- naloxone and substance use disorders. der they thought was cocaine. One (patient A) was in cardiac On January 12, 2019, a similar drug overdose incident was arrest, and two (patients B and C) had opioid toxidrome reported in Chico, California, in which postmortem toxicol- (miosis, respiratory depression, and depressed mental status) ogy testing for one person confirmed fentanyl (1). Fourteen (Table). After spontaneous circulation was reestablished in other persons at the same event were hospitalized with opioid patient A, he was admitted to the intensive care unit, where toxidrome and later released. They reported thinking they he was pronounced brain-dead 3 days later. Patients B and C were snorting cocaine,** but confirmatory toxicology results responded to naloxone, but repeated dosing was required to are unavailable. -

Medications to Treat Opioid Use Disorder Research Report
Research Report Revised Junio 2018 Medications to Treat Opioid Use Disorder Research Report Table of Contents Medications to Treat Opioid Use Disorder Research Report Overview How do medications to treat opioid use disorder work? How effective are medications to treat opioid use disorder? What are misconceptions about maintenance treatment? What is the treatment need versus the diversion risk for opioid use disorder treatment? What is the impact of medication for opioid use disorder treatment on HIV/HCV outcomes? How is opioid use disorder treated in the criminal justice system? Is medication to treat opioid use disorder available in the military? What treatment is available for pregnant mothers and their babies? How much does opioid treatment cost? Is naloxone accessible? References Page 1 Medications to Treat Opioid Use Disorder Research Report Discusses effective medications used to treat opioid use disorders: methadone, buprenorphine, and naltrexone. Overview An estimated 1.4 million people in the United States had a substance use disorder related to prescription opioids in 2019.1 However, only a fraction of people with prescription opioid use disorders receive tailored treatment (22 percent in 2019).1 Overdose deaths involving prescription opioids more than quadrupled from 1999 through 2016 followed by significant declines reported in both 2018 and 2019.2,3 Besides overdose, consequences of the opioid crisis include a rising incidence of infants born dependent on opioids because their mothers used these substances during pregnancy4,5 and increased spread of infectious diseases, including HIV and hepatitis C (HCV), as was seen in 2015 in southern Indiana.6 Effective prevention and treatment strategies exist for opioid misuse and use disorder but are highly underutilized across the United States. -

Approach to the Poisoned Patient
PED-1407 Chocolate to Crystal Methamphetamine to the Cinnamon Challenge - Emergency Approach to the Intoxicated Child BLS 08 / ALS 75 / 1.5 CEU Target Audience: All Pediatric and adolescent ingestions are common reasons for 911 dispatches and emergency department visits. With greater availability of medications and drugs, healthcare professionals need to stay sharp on current trends in medical toxicology. This lecture examines mind altering substances, initial prehospital approach to toxicology and stabilization for transport, poison control center resources, and ultimate emergency department and intensive care management. Pediatric Toxicology Dr. James Burhop Pediatric Emergency Medicine Children’s Hospital of the Kings Daughters Objectives • Epidemiology • History of Poisoning • Review initial assessment of the child with a possible ingestion • General management principles for toxic exposures • Case Based (12 common pediatric cases) • Emerging drugs of abuse • Cathinones, Synthetics, Salvia, Maxy/MCAT, 25I, Kratom Epidemiology • 55 Poison Centers serving 295 million people • 2.3 million exposures in 2011 – 39% are children younger than 3 years – 52% in children younger than 6 years • 1-800-222-1222 2011 Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System Introduction • 95% decline in the number of pediatric poisoning deaths since 1960 – child resistant packaging – heightened parental awareness – more sophisticated interventions – poison control centers Epidemiology • Unintentional (1-2 -

Benzodiazepine & Alcohol Co-Ingestion
The Journal of COLLEGIATE EMERGENCY MEDICAL SERVICES ISSN: 2576-3687 (Print) 2576-3695 (Online) Journal Website: https://www.collegeems.com Benzodiazepine & Alcohol Co-Ingestion: Implications for Collegiate-Based Emergency Medical Services David Goroff & Alexander Farinelli Keywords: alcohol, benzodiazepines, collegiate-based emergency medical services, toxicology Citation (AMA Style): Goroff D, Farinelli A. Benzodiazepine and Alcohol Co-In- gestion. J Coll Emerg Med Serv. 2018; 1(2): 19-23. https://doi.org/10.30542/ JCEMS.2018.01.02.04 Electronic Link: https://doi.org/10.30542/JCEMS.2018.01.02.04 Published Online: August 8, 2018 Published in Print: August 13, 2018 (Volume 1: Issue 2) Copyright: © 2018 Goroff & Farinelli. This is an OPEN ACCESS article distributed under the terms of the Creative Commons Attribu- tion 4.0 International (CC BY 4.0) License, which permits unrestrict- ed use, distribution, and reproduction in any medium, provided the original author and source are credited. The full license is available at: https://creativecommons.org/licenses/by/4.0/ CLINICAL REVIEW Benzodiazepine & Alcohol Co-Ingestion: Implications for Collegiate-Based Emergency Medical Services David Goroff, MS, NRP & Alexander Farinelli, BS, NRP ABSTRACT KEYWORDS: alcohol, The co-ingestion of benzodiazepines and alcohol presents a unique challenge to colle- benzodiazepines, collegiate- giate EMS providers, due to the pharmacological interaction of the two substances and based emergency medical the variable patient presentations. Given the likelihood that collegiate EMS providers services, toxicology will be called to treat a patient who has co-ingested benzodiazepines and alcohol, this Corresponding Author and review discusses the relevant pharmacology, clinical presentation, and treatment of these Author Affiliations:Listed co-ingestion patients. -

Acute Poisoning: Understanding 90% of Cases in a Nutshell S L Greene, P I Dargan, a L Jones
204 REVIEW Postgrad Med J: first published as 10.1136/pgmj.2004.027813 on 5 April 2005. Downloaded from Acute poisoning: understanding 90% of cases in a nutshell S L Greene, P I Dargan, A L Jones ............................................................................................................................... Postgrad Med J 2005;81:204–216. doi: 10.1136/pgmj.2004.024794 The acutely poisoned patient remains a common problem Paracetamol remains the most common drug taken in overdose in the UK (50% of intentional facing doctors working in acute medicine in the United self poisoning presentations).19 Non-steroidal Kingdom and worldwide. This review examines the initial anti-inflammatory drugs (NSAIDs), benzodiaze- management of the acutely poisoned patient. Aspects of pines/zopiclone, aspirin, compound analgesics, drugs of misuse including opioids, tricyclic general management are reviewed including immediate antidepressants (TCAs), and selective serotonin interventions, investigations, gastrointestinal reuptake inhibitors (SSRIs) comprise most of the decontamination techniques, use of antidotes, methods to remaining 50% (box 1). Reductions in the price of drugs of misuse have led to increased cocaine, increase poison elimination, and psychological MDMA (ecstasy), and c-hydroxybutyrate (GHB) assessment. More common and serious poisonings caused toxicity related ED attendances.10 Clinicians by paracetamol, salicylates, opioids, tricyclic should also be aware that severe toxicity can result from exposure to non-licensed pharmaco- -

Safety Notice 009/20 Acetylfentanyl and Fentanyl In
Safety Notice 009/20 Acetylfentanyl and fentanyl in non-opioid illicit drugs 16 October 2020 Background Distributed to: A cluster of hospitalisations due to unexpected opioid toxicity recently occured on • Chief Executives the Central Coast of NSW. Acetylfentanyl and fentanyl are circulating in NSW and • Directors of Clinical Governance may be misrepresented as or be adulterants in illicit cocaine or ketamine. • Director Regulation and Acetylfentanyl has a similar potency to fentanyl, both may cause serious harm and Compliance Unit death. People who do not use opioids regularly (‘opioid naïve’) may be unintentionally exposed and are at high risk of overdose. Even people who Action required by: regularly use opioids are at risk due to the relatively high potency of fentanyl and • Chief Executives acetylfentanyl. • Directors of Clinical Changes in illicit drug use in 2020, as well as variations in purity and alternative Governance ingredients, may be associated with unusual presentations and overdose. • Director Regulation and Compliance Unit Case management • We recommend you also Have a high index of suspicion for illicit fentanyl and fentanyl analogues in inform: suspected opioid overdose. This includes people who deny opioid use or • Drug and Alcohol Directors report use of other non-opioid illicit drugs including ketamine or stimulants and staff such as cocaine, but who present clinically with signs of an opioid • All Service Directors overdose. • Emergency Department • Intensive Care Unit • Airway management, oxygenation, and ventilation support take precedence • Toxicology Units over naloxone, where appropriate. • Ambulance • Cases may require titrated doses of naloxone with a higher total dose of • All Toxicology Staff 800 micrograms or more.