INNER EAR DISORDERS Ear36 (1)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
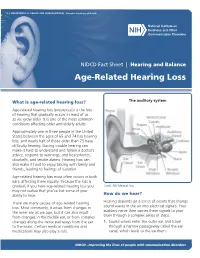
Age-Related Hearing Loss
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES ∙ National Institutes of Health NIDCD Fact Sheet | Hearing and Balance Age-Related Hearing Loss What is age-related hearing loss? The auditory system Age-related hearing loss (presbycusis) is the loss of hearing that gradually occurs in most of us as we grow older. It is one of the most common conditions affecting older and elderly adults. Approximately one in three people in the United States between the ages of 65 and 74 has hearing loss, and nearly half of those older than 75 have difficulty hearing. Having trouble hearing can make it hard to understand and follow a doctor’s advice, respond to warnings, and hear phones, doorbells, and smoke alarms. Hearing loss can also make it hard to enjoy talking with family and friends, leading to feelings of isolation. Age-related hearing loss most often occurs in both ears, affecting them equally. Because the loss is gradual, if you have age-related hearing loss you Credit: NIH Medical Arts may not realize that you’ve lost some of your ability to hear. How do we hear? There are many causes of age-related hearing Hearing depends on a series of events that change loss. Most commonly, it arises from changes in sound waves in the air into electrical signals. Your the inner ear as we age, but it can also result auditory nerve then carries these signals to your from changes in the middle ear, or from complex brain through a complex series of steps. changes along the nerve pathways from the ear 1. -

Assessment of Bone Conduction Thresholds After Surgical Treatment in Patients with Labyrinthine Fistula
Turkish Archives of Otorhinolaryngology Turk Arch Otorhinolaryngol 2018; 56(2): 89-94 89 Türk Otorinolarengoloji Arşivi Assessment of Bone Conduction Thresholds After Surgical Treatment in Patients with Labyrinthine Fistula Müzeyyen Yıldırım Baylan1 , Ümit Yılmaz1 , Zeki Akkuş2 , İsmail Topçu1 1Department of Otorhinolaryngology, Dicle University School of Medicine, Diyarbakır, Turkey Original Investigation 2Department of Biostatistics, Dicle University School of Medicine, Diyarbakır, Turkey Abstract Objective: This study aimed to analyze the bone con- years. In the post-operative period, it was possib- duction thresholds before and after surgery in chronic le to conduct audiological follow-up on 20 patients. otitis media patients with cholesteatoma who had In these follow-ups, 16 patients showed no change labyrinthine fistula and whose cholesteatoma matrix in bone conduction thresholds, two patients showed had been completely cleaned. worsening, and two showed improvement. When Methods: The study was performed between 2013 pre- and post-operative bone conduction thresholds to 2017 with 23 chronic otitis media patients who at each frequency were compared separately, no sig- had labyrinthine fistula with cholesteatoma and who were operated at the Department of Otorhinolar- nificant difference was found (p=0.937). No statis- yngology of Dicle University School of Medicine. tically significant difference was found between the Patients were assessed by anamnesis and examina- pre- and post-operative means at the four frequencies tion and when necessary, by temporal computerized (p=0.712). tomography and diffusion magnetic resonance ima- Conclusion: In this study, we found that to reduce ging. Bone conduction thresholds at frequencies of complications relating to cholesteatoma, it might be 500, 1000, 2000, and 4000 Hz were determined by audiometric examination and they were compared necessary to completely remove the matrix especially before and after surgery. -
HEARING LOSS, DEAFNESS Ear32 (1)
HEARING LOSS, DEAFNESS Ear32 (1) Hearing Loss, Deafness Last updated: May 11, 2019 CLASSIFICATION, DIAGNOSIS ................................................................................................................. 1 METHODS OF COMMUNICATION FOR DEAF ............................................................................................ 2 MANAGEMENT ......................................................................................................................................... 2 HEARING AIDS (S. AMPLIFICATION) ....................................................................................................... 2 COCHLEAR IMPLANTS ............................................................................................................................ 2 AUDITORY BRAINSTEM IMPLANTS .......................................................................................................... 4 PREVENTION .......................................................................................................................................... 4 PEDIATRIC HEARING DEFICITS ............................................................................................................... 4 ETIOLOGY .............................................................................................................................................. 4 DIAGNOSIS ............................................................................................................................................. 6 TREATMENT .......................................................................................................................................... -

Pediatric Sensorineural Hearing Loss, Part 2: Syndromic And
Published May 19, 2011 as 10.3174/ajnr.A2499 Pediatric Sensorineural Hearing Loss, Part 2: REVIEW ARTICLE Syndromic and Acquired Causes B.Y. Huang SUMMARY: This article is the second in a 2-part series reviewing neuroimaging in childhood SNHL. C. Zdanski Previously, we discussed the clinical work-up of children with hearing impairment, the classification of inner ear malformations, and congenital nonsyndromic causes of hearing loss. Here, we review and M. Castillo illustrate the most common syndromic hereditary and acquired causes of childhood SNHL, with an emphasis on entities that demonstrate inner ear abnormalities on cross-sectional imaging. Syndromes discussed include BOR syndrome, CHARGE syndrome, Pendred syndrome, Waardenburg syndrome, and X-linked hearing loss with stapes gusher. We conclude the article with a review of acquired causes of childhood SNHL, including infections, trauma, and neoplasms. ABBREVIATIONS: BOR ϭ branchio-oto-renal; CISS ϭ constructive interference in steady state; IAC ϭ internal auditory canal; NF-2 ϭ neurofibromatosis type II; SCC ϭ semicircular canal; SNHL ϭ sensorineural hearing loss; T1WI ϭ T1-weighted image; T2 WI ϭ T2-weighted image he estimated prevalence of SNHL in patients younger than Table 1: Selected hereditary syndromes commonly associated with 1 T18 years of age is 6 per 1000, making it one of the leading SNHL causes of childhood disability and a common reason for oto- Inner Ear Malformations on Inner Ear Malformations laryngology referrals. Cross-sectional imaging is now rou- Imaging Not Common on Imaging tinely performed in these patients because it provides impor- Alagille syndrome Alport syndrome tant information about potential etiologies for hearing loss, Branchio-oto-renal syndrome Biotinidase deficiency defines the anatomy of the temporal bone and the central au- CHARGE syndrome Jervell and Lange-Nielsen syndrome ditory pathway, and identifies additional intracranial abnor- Klippel-Feil syndrome Norrie syndrome malities that may require further work-up. -

PRESBYCUSIS Diagnosis and Treatment
Hear the FACTS about PRESBYCUSIS Diagnosis and Treatment NORMAL HEARING What is Presbycusis? FREQUENCY (in Hertz) . A gradual reduction in hearing as we get older, typically affecting both 250 500 1000 2000 4000 8000 -10 ears equally. 0 X 10 X X Common in men and women, with men typically having greater X •X . 20 • •X • • • 30 hearing loss than women of the same age. 40 Typically a greater hearing loss for high frequency sounds than for low 50 . (in dBHL) 60 INTENSITY frequency sounds. 70 80 A treatable condition that can benefit greatly from technological 90 . advances in various amplification or hearing assistance devices, along 100 • Right Ear 110 X Left Ear with counseling on effective communication strategies. PRESBYCUSIS HEARING LOSS What causes Presbycusis? FREQUENCY (in Hertz) . Family history or hereditary factors. 250 500 1000 2000 4000 8000 -10 Changes in the inner ear blood supply related to heart disease, 0 . 10 •X diabetes, high blood pressure and smoking. 20 •X 30 •X A loss of sound sensitivity from cumulative exposure to loud sounds. 40 •X . 50 (in dBHL) 60 INTENSITY X 70 • Symptoms: X 80 • •X 90 . Frequently asking people to repeat what they say, especially in 100 Right Ear “difficult listening places”. 110 X Left Ear . Ability to hear lower-pitched men's voices easier than higher-pitched NORMAL INNER EAR PRESBYCUSIC INNER EAR women’s or children’s voices. People complaining that the TV is being played too loud. Tinnitus, also known as “head noise”, which produces buzzing or ringing sounds in the ear. Diagnosis: . Talk honestly with your Hearing Healthcare Provider about daily hearing problems. -

A Molecular and Genetic Analysis of Otosclerosis
A molecular and genetic analysis of otosclerosis Joanna Lauren Ziff Submitted for the degree of PhD University College London January 2014 1 Declaration I, Joanna Ziff, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. Where work has been conducted by other members of our laboratory, this has been indicated by an appropriate reference. 2 Abstract Otosclerosis is a common form of conductive hearing loss. It is characterised by abnormal bone remodelling within the otic capsule, leading to formation of sclerotic lesions of the temporal bone. Encroachment of these lesions on to the footplate of the stapes in the middle ear leads to stapes fixation and subsequent conductive hearing loss. The hereditary nature of otosclerosis has long been recognised due to its recurrence within families, but its genetic aetiology is yet to be characterised. Although many familial linkage studies and candidate gene association studies to investigate the genetic nature of otosclerosis have been performed in recent years, progress in identifying disease causing genes has been slow. This is largely due to the highly heterogeneous nature of this condition. The research presented in this thesis examines the molecular and genetic basis of otosclerosis using two next generation sequencing technologies; RNA-sequencing and Whole Exome Sequencing. RNA–sequencing has provided human stapes transcriptomes for healthy and diseased stapes, and in combination with pathway analysis has helped identify genes and molecular processes dysregulated in otosclerotic tissue. Whole Exome Sequencing has been employed to investigate rare variants that segregate with otosclerosis in affected families, and has been followed by a variant filtering strategy, which has prioritised genes found to be dysregulated during RNA-sequencing. -
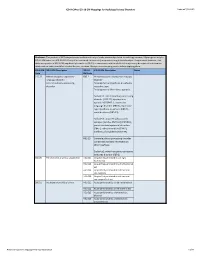
ICD-9/10 Mapping Spreadsheet
ICD-9-CM to ICD-10-CM Mappings for Audiology Related Disorders Updated 7/16/2015 Disclaimer: This product is NOT comprehensive and consists only of codes commonly related to audiology services. Mappings are only to ICD-10-CM codes, not ICD-10-PCS. Every effort was made to accurately map codes using detailed analysis. Keep in mind, however, that while many codes in ICD-9-CM map directly to codes in ICD-10, in some cases, additional clinical analysis may be required to determine which code or codes should be selected for your situation. Always review mapping results before applying them. ICD-9-CM ICD-9-CM Description ICD-10- ICD-10-CM Description Notes Code CM Code 315.32 Mixed receptive-expressive F80.2 Mixed receptive-expressive language language disorder disorder Central auditory processing Developmental dysphasia or aphasia, disorder receptive type Developmental Wernicke's aphasia Excludes1: central auditory processing disorder (H93.25), dysphasia or aphasia NOS (R47.-), expressive language disorder (F80.1), expressive type dysphasia or aphasia (F80.1), word deafness (H93.25) Excludes2: acquired aphasia with epilepsy [Landau-Kleffner] (G40.80-), pervasive developmental disorders (F84.-), selective mutism (F94.0), intellectual disabilities (F70-F79) H93.25 Central auditory processing disorder Congenital auditory imperception Word deafness Excludes1: mixed receptive-epxressive language disorder (F80.2) 380.00 Perichondritis of pinna, unspecified H61.001 Unspecified perichondritis of right external ear H61.002 Unspecified perichondritis -

Vestibular Neuritis and Labyrinthitis
Vestibular Neuritis and DISORDERS Labyrinthitis: Infections of the Inner Ear By Charlotte L. Shupert, PhD with contributions from Bridget Kulick, PT and the Vestibular Disorders Association INFECTIONS Result in damage to inner ear and/or nerve. ARTICLE 079 DID THIS ARTICLE HELP YOU? SUPPORT VEDA @ VESTIBULAR.ORG Vestibular neuritis and labyrinthitis are disorders resulting from an 5018 NE 15th Ave. infection that inflames the inner ear or the nerves connecting the inner Portland, OR 97211 ear to the brain. This inflammation disrupts the transmission of sensory 1-800-837-8428 information from the ear to the brain. Vertigo, dizziness, and difficulties [email protected] with balance, vision, or hearing may result. vestibular.org Infections of the inner ear are usually viral; less commonly, the cause is bacterial. Such inner ear infections are not the same as middle ear infections, which are the type of bacterial infections common in childhood affecting the area around the eardrum. VESTIBULAR.ORG :: 079 / DISORDERS 1 INNER EAR STRUCTURE AND FUNCTION The inner ear consists of a system of fluid-filled DEFINITIONS tubes and sacs called the labyrinth. The labyrinth serves two functions: hearing and balance. Neuritis Inflamation of the nerve. The hearing function involves the cochlea, a snail- shaped tube filled with fluid and sensitive nerve Labyrinthitis Inflamation of the labyrinth. endings that transmit sound signals to the brain. Bacterial infection where The balance function involves the vestibular bacteria infect the middle organs. Fluid and hair cells in the three loop-shaped ear or the bone surrounding semicircular canals and the sac-shaped utricle and Serous the inner ear produce toxins saccule provide the brain with information about Labyrinthitis that invade the inner ear via head movement. -

Vascular Supply of the Human Spiral Ganglion: Novel Three
www.nature.com/scientificreports Corrected: Publisher Correction OPEN Vascular Supply of the Human Spiral Ganglion: Novel Three- Dimensional Analysis Using Synchrotron Phase-Contrast Imaging and Histology Xueshuang Mei1,2*, Rudolf Glueckert3, Annelies Schrott-Fischer3, Hao Li1, Hanif M. Ladak4,6, Sumit K. Agrawal5,6 & Helge Rask-Andersen1,6* Human spiral ganglion (HSG) cell bodies located in the bony cochlea depend on a rich vascular supply to maintain excitability. These neurons are targeted by cochlear implantation (CI) to treat deafness, and their viability is critical to ensure successful clinical outcomes. The blood supply of the HSG is difcult to study due to its helical structure and encasement in hard bone. The objective of this study was to present the frst three-dimensional (3D) reconstruction and analysis of the HSG blood supply using synchrotron radiation phase-contrast imaging (SR-PCI) in combination with histological analyses of archival human cochlear sections. Twenty-six human temporal bones underwent SR-PCI. Data were processed using volume-rendering software, and a representative three-dimensional (3D) model was created to allow visualization of the vascular anatomy. Histologic analysis was used to verify the segmentations. Results revealed that the HSG is supplied by radial vascular twigs which are separate from the rest of the inner ear and encased in bone. Unlike with most organs, the arteries and veins in the human cochlea do not follow the same conduits. There is a dual venous outfow and a modiolar arterial supply. This organization may explain why the HSG may endure even in cases of advanced cochlear pathology. Human inner ear function relies on microcirculation derived from vessels in the internal auditory canal (IAC). -

Spectrum of Third Window Abnormalities: Semicircular Canal Dehiscence and Beyond
Published August 25, 2016 as 10.3174/ajnr.A4922 REVIEW ARTICLE Spectrum of Third Window Abnormalities: Semicircular Canal Dehiscence and Beyond X M.-L. Ho, X G. Moonis, X C.F. Halpin, and X H.D. Curtin ABSTRACT SUMMARY: Third window abnormalities are defects in the integrity of the bony structure of the inner ear, classically producing sound-/ pressure-induced vertigo (Tullio and Hennebert signs) and/or a low-frequency air-bone gap by audiometry. Specific anatomic defects include semicircular canal dehiscence, perilabyrinthine fistula, enlarged vestibular aqueduct, dehiscence of the scala vestibuli side of the cochlea, X-linked stapes gusher, and bone dyscrasias. We discuss these various entities and provide key examples from our institutional teaching file with a discussion of symptomatology, temporal bone CT, audiometry, and vestibular-evoked myogenic potentials. ABBREVIATIONS: EVAS ϭ enlarged vestibular aqueduct syndrome; SSCCD ϭ superior semicircular canal dehiscence hird window abnormalities are defects in the integrity of the ing effects decrease air conduction. The 2 physiologic windows Tbony structure of the inner ear, first described by Minor et al between the middle and inner ear are the oval window, which in 1998.1 In 2008, Merchant and Rosowski2 proposed a universal transmits vibrations from the auditory ossicles, and the round theory for the underlying mechanism of hearing loss accompany- window of the cochlea. With air conduction, there is physiologic ing these defects. Normal sound conduction is transmitted entrainment of the oval and round windows due to coupling by through the oval and round windows, which serve as fluid inter- incompressible perilymph. Pressure differences between the co- faces between air in the middle ear and perilymphatic fluid spaces chlear perilymphatic spaces activate hair cells and create the per- of the inner ear. -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Alzheimer's Disease, Hearing Loss, Presbycusis, Tinnitus, Older Adults
International Journal of Psychology and Behavioral Sciences 2018, 8(5): 77-80 DOI: 10.5923/j.ijpbs.20180805.01 Alzheimer’s Disease and Hearing Loss among Older Adults: A Literature Review Fereshteh Bagheri1, Vahidreza Borhaninejad2, Vahid Rashedi3,4,* 1Department of Audiology, School of Rehabilitation Sciences, Babol University of Medical Sciences, Mazandaran, Iran 2Social Determinants of Health Research Center, Institute for Futures Studies in Health, Kerman University of Medical Sciences Kerman, Iran 3School of Behavioural Sciences and Mental Health (Tehran Institute of Psychiatry), Iran University of Medical Sciences, Tehran, Iran 4Iranian Research Centre on Aging, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran Abstract Older adults with hearing loss are more likely to develop Alzheimer's disease (AD) or dementia compared to those with normal hearing. Hearing loss can be consecutive to presbycusis and/or central auditory dysfunction. The current study reviewed the literature concerning the relationship between hearing loss and AD among older adults. Articles included in this review were identified through a search of the databases PubMed, Medline, Scopus, Google Scholar, and Scientific Information Database (SID) using the search terms Alzheimer’s disease, dementia, presbycusis, hearing loss, and hearing impairment. The literature search was restricted to the years 1989 to 2018 and to articles published in the English language. Of 54 primary articles, 38 potentially eligible articles were reviewed. Although cognitive decline has been shown to be slowed by the use of hearing aids in older adults, a few studies have investigated the effects of other factors such as presbycusis-related tinnitus and length of use of hearing aids by older adults.