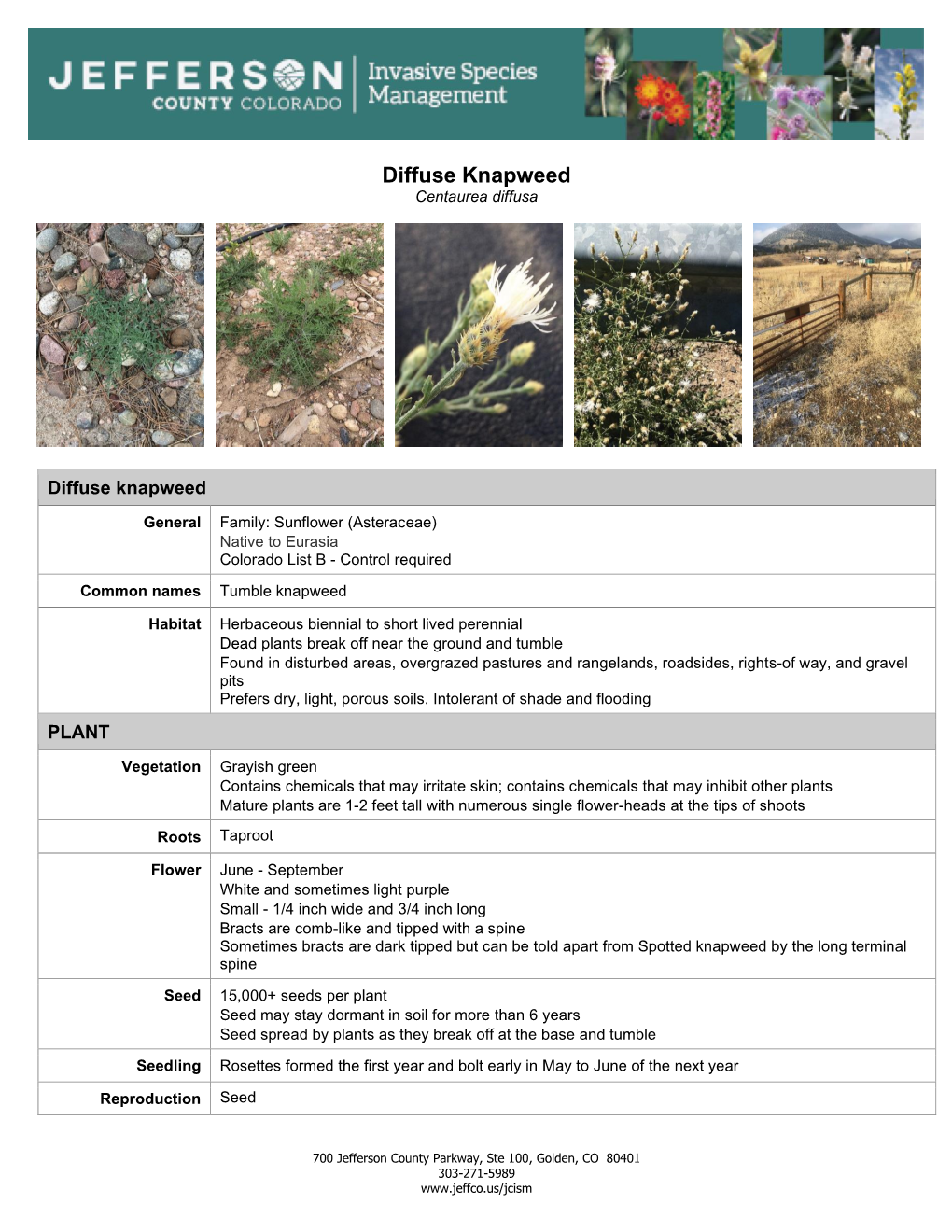
Information Sheet on Diffuse Knapweed (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Assessing Population Sizes, Biological Potential and Mass
1. ASSESSING POPULATION SIZES, BIOCONTROL POTENTIAL AND MASS PRODUCTION OF THE ROOT BORING MOTH AGAPETA ZOEGANA FOR AREWIDE IMPLEMENTATION AND MONITORING OF SPOTTED KNAPWEED BIOCONTROL 2. PRINCIPAL INVESTIGATORS: Mark Schwarzländer, PSES Department, University of Idaho, 875 Perimeter DR MS 2339, Moscow, ID 83844-2339, (208) 885-9319, FAX (208)885- 7760, [email protected]; Joseph Milan, USDI Bureau of Land Management, 3948 Development Ave., Boise, ID 83705, (208) 384-3487, FAX (208) 384-3326, [email protected]; Paul Brusven, Nez Perce Tribe Bio-Control Center, P.O. Box 365, 22776 Beaver Road, Lapwai, ID 83540, (208) 843-9374, FAX (208) 843-9373, [email protected] 3. COOPERATORS: Dr. Hariet Hinz (CABI Switzerland), Dr. Urs Schaffner (CABI Switzerland, Dr. Sanford Eigenbrode (University of Idaho), Dr. Heinz Müller-Schärer (University of Fribourg, Switzerland), Brian Marschmann (USDA APHIS PPQ State Director, Idaho), Dr. Rich Hansen (USDA APHIS CPHST, Ft. Collins, Colorado), John (Lewis) Cook (USDI BIA Rocky Mountain Region, Billings, Montana), Dr. John Gaskin (USDA ARS NPARL, Sidney, Montana), Idaho County Weed Superintendents and Idaho-based USFS land managers. BCIP CONTACT: Carol Randall, USFS Northern and Intermountain Regions, 2502 E Sherman Ave, Coeur d'Alene, ID 83814, (208) 769-3051, (208) 769-3062, [email protected] 4. REQUESTED FUNDS: USFS $100,000 (Year 1: $34,000; Year 2: $33,000; and Year 3: $33,000), Project Leveraging: University of Idaho $124,329. 5. EXECUTIVE SUMMARY: 1) The current status of ecological research suggests that albeit having some impact on spotted knapweed, both, A. zoegana and C. achates have stronger negative effects on native grasses, thus indirectly benefiting one of most devastating invasive plants in the U.S. -

Montana Knapweeds
Biology, Ecology and Management of Montana Knapweeds EB0204 revised August 2017 Celestine Duncan, Consultant, Weed Management Services, Helena, MT Jim Story, Research Professor, retired, MSU Western Ag Research Center, Corvallis, MT Roger Sheley, former MSU Extension Weed Specialist, Bozeman, MT revised by Hilary Parkinson, former MSU Research Associate, and Jane Mangold, MSU Extension Invasive Plant Specialist Table of Contents Plant Biology . 3 SpeedyWeed ID . 5 Ecology . 4 Habitat . 4 Spread and Establishment Potential . 6 Damage Potential . 7 Origins, Current Status and Distribution . 8 Management Alternatives . 8 Prevention . 8 Mechanical Control . .9 Cultural Control . .10 Biological Control . .11 Chemical Control . .14 Integrated Weed Management (IWM) . 16 Additional Resources . 17 Acknowledgements . .19 COVER PHOTOS large - spotted knapweed by Marisa Williams, University of Arkansas, Fayetteville, bugwood.org top inset - diffuse knapweed by Cindy Roche, bugwood.org bottom inset - Russain knapweed by Steve Dewey, Utah State University, bugwood.org Any mention of products in this publication does not constitute a recommendation by Montana State University Extension. It is a violation of Federal law to use herbicides in a manner inconsistent with their labeling. Copyright © 2017 MSU Extension The U.S. Department of Agriculture (USDA), Montana State University and Montana State University Extension prohibit discrimination in all of their programs and activities on the basis of race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, and marital and family status. Issued in furtherance of cooperative extension work in agriculture and home economics, acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, Jeff Bader, Director of Extension, Montana State University, Bozeman, MT 59717. -

Weed Biocontrol: Extended Abstracts from the 1997 Interagency Noxious-Weed Symposium
Weed Biocontrol: Extended Abstracts from the 1997 Interagency Noxious-Weed Symposium Dennis Isaacson Martha H. Brookes Technical Coordinators U.S. Department of Agriculture Forest Service Forest Health Technology Enterprise Team Morgantown, WV and Oregon Department of Agriculture Salem, OR June 1999 ACKNOWLEDGMENTS Many of the tasks of organizing a symposium such as this — and there are many — are not obvious, and, if they are handled well, the effort that goes into them can easily be overlooked. Sherry Kudna of the Oregon Department of Agriculture Weed Control staff managed most of the arrangements and took care of many, many details, which helped the symposium run smoothly. We truly appreciate her many contributions. We also acknowledge the contributions of the presenters. They not only organized their own presentations and manuscripts, but also assisted with reviewing drafts of each other’s papers in the proceedings. Several of the presenters also covered their own expenses. Such dedication speaks well of their commitment to improving the practice of weed biocontrol. Both the Oregon State Office of the Bureau of Land Management and the USDA Forest Service made major contributions to supporting the symposium. Although several individuals from both organizations provided assistance, we especially note the encouragement and advice of Bob Bolton, Oregon Bureau of Land Management Weed Control Coordinator, and the willingness to help and financial support for publishing this document from Richard C. Reardon, Biocontrol/Biopesticides Program Manager, USDA Forest Service's Forest Health Technology Enterprise Team, Morgantown, WV. We thank Tinathan Coger for layout and design and Patricia Dougherty for printing advice and coordination of the manuscript We also thank Barbra Mullin, Montana State Department of Agriculture, who delivered the keynote address; Tami Lowry, Pacific Northwest Research Station, Corvallis, who helped format the document; and Eric Coombs, who provided the photographs of weeds and agents that convey the concepts of weed biocontrol. -

A Potential Collection Method for Agapeta Zoegana (Lepidoptera: Cochylidae), a Knapweed·Root·Feeding Moth Sheila M
J. ENTOMOL Soc. BRIT. COLUMBIA 86 (1989), SEPT. 3D, 1989 55 A POTENTIAL COLLECTION METHOD FOR AGAPETA ZOEGANA (LEPIDOPTERA: COCHYLIDAE), A KNAPWEED·ROOT·FEEDING MOTH SHEILA M. FITZPATRICK AGRICULWRE CANADA REsEARCH STATION 6660 N.W. MARINE DRIVE VANCOUVER, B.C. V6T lX2 ABSTRACT This paper describes a method for collecting living, undamaged Agapeta zoegana (L.) moths, especially recently mated females. The objective was to gather this potential biological control agent for subsequent distribution to land infested with Icnapweeds (CenJaurea spp.) Sweep-netting and baiting techniques were inappropriate collection methods, because the moths were delicate and did not appear to forage. The moths did not move to the plant tops at particular temperatures or times of day and therefore could not easily be collected by aspiration. However, males and virgin and mated females within large field cages were attracted to UV light and, during their daily period of reproductive activity from dusk to midnight, could be collected in a Heliothis trap (Sentry) illuminated by a blacklight. In the open, neither this method nor a mobile blacklight technique were successful in 1988, but both warrant further work. Results are discussed in the context of A. zoegana establishment in B.C. INTRODUCTION Diffuse (Centaurea diffusa Lam.) and spotted (c. maculosa Lam.) knapweed, introduced from Europe in the early 1900's, pose a serious threat to range- and pasture-hinds in B.C. (Cranston, 1980). The knapweeds outcompete native forage species on disturbed or over grazed sites, and are of low value as forage (Harris and Myers 1984). Chemical control of knapweed in most areas is neither economically practical nor environmentally desirable (Cranston 1980). -

13 SPOTTED KNAPWEED PEST STATUS of WEED Nature Of
In: Van Driesche, R., et al., 2002, Biological Control of Invasive Plants in the Eastern United States, USDA Forest Service Publication FHTET-2002-04, 413 p. 13 SPOTTED KNAPWEED J. Story Montana State University, Western Agricultural Research Center, Corvallis, Montana, USA runoff and soil sedimentation (Lacey et al., 1989), and PEST STATUS OF WEED lowers plant diversity (Tyser and Key, 1988). Spot- Spotted knapweed, Centaurea maculosa Lamarck, is ted knapweed produces an allelopathic compound a purple-flowered, herbaceous, perennial weed, liv- that reduces germination of some grass species ing three to five years on average. It infests semiarid (Kelsey and Locken, 1987). range lands in the western United States and road- Geographical Distribution sides and fields in the eastern part of the country. Infested areas are dominated by the plant, reducing Spotted knapweed is native to Europe and western their grazing value and suppressing native plant com- Asia but has become widespread in parts of the munities. The plant, originally from Central Asia, has United States and Canada. The plant occurs through- been in North America for over 120 years. out the United States except for Alaska, Texas, Okla- homa, Mississippi, and Georgia (USDA, NRCS, Nature of Damage 2001). The plant is a serious invader of rangeland in Economic damage. Spotted knapweed is a serious the Rocky Mountain region. In Montana alone, the problem on rangeland, especially in the western plant infests an estimated 1.9 million ha of rangeland United States. Bucher (1984) estimated that an and pasture (Lacey, 1989). In Canada, the plant is 800,000 ha infestation in Montana was causing $4.5 abundant in British Columbia, and is common in million in annual forage losses, and that invasion of Ontario, Quebec, and the Maritimes (Watson and 13.6 million ha of vulnerable rangeland in Montana Renney, 1974). -

Scientific Names of Pest Species in Tortricidae (Lepidoptera)
RESEARCH Scientific Names of Pest Species in Tortricidae (Lepidoptera) Frequently Cited Erroneously in the Entomological Literature John W. Brown Abstract. The scientific names of several pest species in the moth meate the literature. For example, the subfamilial designation for family Tortricidae (Lepidoptera) frequently are cited erroneously in Olethreutinae (rather than Olethreutidae) was slow to be accepted contemporary entomological literature. Most misuse stems from the for many years following Obraztsov’s (1959) treatment of the group. fact that many proposed name changes appear in systematic treat- They even appear at both taxonomic levels (i.e., Olethreutinae and ments that are not seen by most members of the general entomologi- Olethreutidae) in different papers in the same issue of the Canadian cal community. Also, there is resistance among some entomologists Entomologist in the 1980s! (Volume 114 (6), 1982) Olethreutinae to conform to recently proposed changes in the scientific names of gradually was absorbed into the North America literature, espe- well-known pest species. Species names discussed in this paper are cially following publication of the Check List of the Lepidoptera Brazilian apple leafroller, Bonagota salubricola (Meyrick); western of America North of Mexico (Hodges 1983), which has served as a black-headed budworm, Acleris gloverana (Walsingham); and green standard for more than 20 years. budworm, Choristoneura retiniana (Walsingham). Generic names During preparation of a world catalog of Tortricidae (Brown discussed include those for false codling moth, Thaumatotibia leu- 2005), it became obvious to me that several taxonomically correct cotreta (Meyrick); grape berry moth, Paralobesia viteana (Clemens); combinations of important pest species were not in common use in pitch twig moth, Retinia comstockiana (Fernald); codling moth, the entomological literature. -

Forest Health Technology Enterprise Team Biological Control of Invasive
Forest Health Technology Enterprise Team TECHNOLOGY TRANSFER Biological Control Biological Control of Invasive Plants in the Eastern United States Roy Van Driesche Bernd Blossey Mark Hoddle Suzanne Lyon Richard Reardon Forest Health Technology Enterprise Team—Morgantown, West Virginia United States Forest FHTET-2002-04 Department of Service August 2002 Agriculture BIOLOGICAL CONTROL OF INVASIVE PLANTS IN THE EASTERN UNITED STATES BIOLOGICAL CONTROL OF INVASIVE PLANTS IN THE EASTERN UNITED STATES Technical Coordinators Roy Van Driesche and Suzanne Lyon Department of Entomology, University of Massachusets, Amherst, MA Bernd Blossey Department of Natural Resources, Cornell University, Ithaca, NY Mark Hoddle Department of Entomology, University of California, Riverside, CA Richard Reardon Forest Health Technology Enterprise Team, USDA, Forest Service, Morgantown, WV USDA Forest Service Publication FHTET-2002-04 ACKNOWLEDGMENTS We thank the authors of the individual chap- We would also like to thank the U.S. Depart- ters for their expertise in reviewing and summariz- ment of Agriculture–Forest Service, Forest Health ing the literature and providing current information Technology Enterprise Team, Morgantown, West on biological control of the major invasive plants in Virginia, for providing funding for the preparation the Eastern United States. and printing of this publication. G. Keith Douce, David Moorhead, and Charles Additional copies of this publication can be or- Bargeron of the Bugwood Network, University of dered from the Bulletin Distribution Center, Uni- Georgia (Tifton, Ga.), managed and digitized the pho- versity of Massachusetts, Amherst, MA 01003, (413) tographs and illustrations used in this publication and 545-2717; or Mark Hoddle, Department of Entomol- produced the CD-ROM accompanying this book. -

Plant Conservation Alliance®S Alien Plant Working Group
FACT SHEET: SPOTTED KNAPWEED Spotted Knapweed Centaurea stoebe L. ssp. micranthos (Gugler) Hayek Sunflower family (Asteraceae) NATIVE RANGE Central Europe, east to central Russia, Caucasia, and western Siberia DESCRIPTION Spotted knapweed, previously known as Centaurea biebersteinii, is a biennial or short-lived perennial. Its name is derived from the spots formed by black margins on the flower bract tips. Spotted knapweed typically forms a basal rosette of leaves in its first year and flowers in subsequent years. Rosette leaves are approximately 8 inches long by 2 inches wide, borne on short stalks, and deeply lobed once or twice on both sides of the center vein, with lobes oblong and wider toward the tip. The taproot is stout and deep. Flowering stems are erect, 8 to 50 inches tall, branched above the middle, and sparsely to densely hairy. Stem leaves alternate along the stem, are unstalked, and may be slightly lobed, or linear and unlobed. Leaf size decreases towards the tip of the stem. Flowers are purple to pink, rarely white, with 25 to 35 flowers per head. Plants bloom from June to October, and flower heads usually remain on the plant. Flower heads are oblong or oval shaped, ¼ inch wide and ½ inch across, and are single or borne in clusters of two or three at the branch ends. Leaf like bracts surrounding the base of the flower head are oval and yellow green, becoming brown near the base. The margins of these bracts have a soft spine like fringe, with the center spine being shorter than the lateral spines. The brown, oval seeds are 1/16 to 1/8 inch long, with pale longitudinal lines and a short fringe on one end. -

Optimal Site Release Strategies and Impact of Biological Control Agents on Spotted Knapweed, Centaurea Maculosa
University of Massachusetts Amherst ScholarWorks@UMass Amherst Masters Theses 1911 - February 2014 2000 Optimal site release strategies and impact of biological control agents on spotted knapweed, Centaurea maculosa. Sheryl Clark University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/theses Clark, Sheryl, "Optimal site release strategies and impact of biological control agents on spotted knapweed, Centaurea maculosa." (2000). Masters Theses 1911 - February 2014. 3080. Retrieved from https://scholarworks.umass.edu/theses/3080 This thesis is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Masters Theses 1911 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. OPTIMAL SITE RELEASE STRATEGIES AND IMPACT OF BIOLOGICAL CONTROL AGENTS ON SPOTTED KNAPWEED, CENTAUREA MACULOSA A Thesis Presented by SHERYL CLARK Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of MASTERS OF ENTOMOLOGY September 2000 Department of Entomology OPTIMAL SITE RELEASE STRATEGIES AND IMPACT OF BIOLOGICAL CONTROL AGENTS ON SPOTTED KNAPWEED, CENTAUREA MACULOSA A Thesis Presented by SHERYL CLARK Approved as to style and content by: 4 Joseph Elkinton, Member aL g. John G. Stoffolano, Acting Department Head Department of Entomology ACKNOWLEDGMENTS I would like to thank my thesis committee chair Dr. Roy Van Driesche for all of his contributions and support. Aside from providing advice, guidance, encouragement and support, he believed in my abilities to accomplish this Masters project. I also want to thank Dr. Joseph Elkinton and Dr. -

Laboratory Rearing Procedures for Two Lepidopteran Weed Biocontrol Agents
Scientific Notes 95 LABORATORY REARING PROCEDURES FOR TWO LEPIDOPTERAN WEED BIOCONTROL AGENTS ASHOK RAINA1*, DALE GELMAN2, CURTIS HUBER3 AND NEAL SPENCER4 1Formosan Subterranean Termite Research Unit, USDA-ARS, New Orleans, LA 70124 2Insect Biocontrol Laboratory, USDA-ARS, Beltsville, MD 20705 3Center for Disease Control, Atlanta, GA 30333 4Plant Protection Research Unit, USDA-ARS, Ithaca, NY 14853 *Author for correspondence; e-mail: [email protected] Most noxious weeds infesting rangeland are univoltine, diapausing during the winter months exotic species (DiTomaso 2000). The genus Cen- as 3rd instars (Campobasso et al. 1994; Dunn at taurea which contains several species of knap- al. 1989). A. zoegana, which has six instars, can weed is the most abundant group in the western complete 2-3 generations per year in Europe United States (Skinner et al. 2000). In their na- (Müller et al. 1988), whereas in British Columbia, tive habitat of Eurasia, natural enemies have pre- it is restricted to only one generation (Muir & vented knapweed from becoming an economic Harris 1987). However, there is little information problem (Keane & Crawley 2002). However, in the on the nature of the diapause and no descriptions United States where they were accidentally intro- of rearing methodology for either of these species. duced more than 100 years ago, these weeds, in We report here that both species can be reared the absence of their natural enemies, have repro- from the egg to the adult stage on artificial diet duced unchecked and replaced many of the more and that diapause can be averted or shortened desirable rangeland vegetation. Biological control under our rearing conditions. -

Agapeta Zoegana L
Biological Control 26 (2003) 270–278 www.elsevier.com/locate/ybcon Plant size preference of Agapeta zoegana L. (Lepidoptera: Tortricidae), a root-feeding biological control agent of spotted knapweed L. Smitha,* and J.M. Storyb a USDA-ARS, Western Regional Research Center, 800 Buchanan Street, Albany, CA 94710, USA b Montana Agricultural Experiment Station, Western Agricultural Research Center, Corvallis, MT 59828, USA Received 26 December 2001; accepted 22 October 2002 Abstract Agapeta zoegana L. (Lepidoptera: Tortricidae) is an oligophagous herbivore that was introduced to North America as a bio- logical control agent of spotted knapweed, Centaurea stoebe L. subsp. micranthos (Gugler) Hayek (often called Centaurea maculosa Lam.). Spotted knapweed is a perennial plant that usually increases in size each year. A previous field study reported that more larvae were found on larger plants and that infested plants tended to be larger than uninfested ones. Precisely quantifying the size- specific attack rate can help us model the impact of this agent on the weed population and better understand the interspecific in- teractions to improve the effectiveness of biological control. Field data were analyzed to determine the relative preference of attack for each size class of the host plant. Plants were classified based on root diameter at 2 cm below the root crown. Although small plants (<3 mm root diameter) were more abundant in the field population, the highest infestation rates occurred in large plants. ChessonÕs electivity index was generally positive for root diameters >3.5 mm, indicating preferential attack of large plants. Because of its host-size preference, A. zoegana is expected to primarily affect large plants, which is contrary to previous expectations. -

Agapeta Zoegana (Sulphur Knapweed Moth, Yellow-Winged Knapweed Root Moth)
RANGE Operational Field Guide to the propagation and establishment of the bioagent Agapeta zoegana (Sulphur knapweed moth, yellow-winged knapweed root moth) March 1998 The contents of this Field Guide may not be cited in whole or in part without the advance written approval of the Director, Forest Practices Branch, Ministry of Forests, Victoria, British Columbia Information contained in this Field Guide is comprised of fact and field observations as of March 1998. Site specific experiences may vary. Operational Field Guide to the propagation and establishment of the bioagent Agapeta zoegana (Sulphur knapweed moth, yellow-winged knapweed root moth) March 1998 Forest Practices Branch Range Section Noxious Weed Control Program British Columbia Ministry of Forests Agapeta zoegana (Sulphur knapweed moth, yellow-winged knapweed root moth) Operational Field Guide TABLE OF CONTENTS 1. PURPOSE .................................................................................................................. 1 2. INTRODUCTION ...................................................................................................... 1 3. AGAPETA ZOEGANA ................................................................................................ 1 BIOLOGY ............................................................................................................1 RANGE ................................................................................................................3 Native (European) Range ..............................................................................