Biology and Genetics of Reproduction M
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Revised Glossary for AQA GCSE Biology Student Book
Biology Glossary amino acids small molecules from which proteins are A built abiotic factor physical or non-living conditions amylase a digestive enzyme (carbohydrase) that that affect the distribution of a population in an breaks down starch ecosystem, such as light, temperature, soil pH anaerobic respiration respiration without using absorption the process by which soluble products oxygen of digestion move into the blood from the small intestine antibacterial chemicals chemicals produced by plants as a defence mechanism; the amount abstinence method of contraception whereby the produced will increase if the plant is under attack couple refrains from intercourse, particularly when an egg might be in the oviduct antibiotic e.g. penicillin; medicines that work inside the body to kill bacterial pathogens accommodation ability of the eyes to change focus antibody protein normally present in the body acid rain rain water which is made more acidic by or produced in response to an antigen, which it pollutant gases neutralises, thus producing an immune response active site the place on an enzyme where the antimicrobial resistance (AMR) an increasing substrate molecule binds problem in the twenty-first century whereby active transport in active transport, cells use energy bacteria have evolved to develop resistance against to transport substances through cell membranes antibiotics due to their overuse against a concentration gradient antiretroviral drugs drugs used to treat HIV adaptation features that organisms have to help infections; they -

Reproductive Ecology & Sexual Selection
Reproductive Ecology & Sexual Selection REPRODUCTIVE ECOLOGY REPRODUCTION & SEXUAL SELECTION • Asexual • Sexual – Attraction, Courtship, and Mating – Fertilization – Production of Young The Evolutionary Enigma of Benefits of Asex Sexual Reproduction • Sexual reproduction produces fewer reproductive offspring than asexual reproduction, a so-called reproductive handicap 1. Eliminate problem to locate, court, & retain suitable mate. Asexual reproduction Sexual reproduction Generation 1 2. Doubles population growth rate. Female Female 3. Avoid “cost of meiosis”: Generation 2 – genetic representation in later generations isn't reduced by half each time Male 4. Preserve gene pool adapted to local Generation 3 conditions. Generation 4 Figure 23.16 The Energetic Costs of Sexual Reproduction Benefits of Sex • Allocation of Resources 1. Reinforcement of social structure 2. Variability in face of changing environment. – why buy four lottery tickets w/ the same number on them? Relative benefits: Support from organisms both asexual in constant & sexual in changing environments – aphids have wingless female clones & winged male & female dispersers – ciliates conjugate if environment is deteriorating Heyer 1 Reproductive Ecology & Sexual Selection Simultaneous Hermaphrodites TWO SEXES • Advantageous if limited mobility and sperm dispersal and/or low population density • Guarantee that any member of your species encountered is the • Conjugation “right” sex • Self fertilization still provides some genetic variation – Ciliate protozoans with + & - mating -

Cellular Reproduction
572 14 Cellular Reproduction 14.1 The Cell Cycle According to the third tenet of the cell theory, new cells 14.2 M Phase: Mitosis and Cytokinesis originate only from other living cells. The process by which this cell division 14.3 Meiosis occurs is called . For a multicellular organism, such as a human or an oak tree, countless divisions of a single-celled THE HUMAN PER SP ECTIVE: Meiotic Nondisjunction zygote produce an organism of astonishing cellular complexity and Its Consequences and organization. Cell division does not stop with the formation of EXPERIMENTAL PATHWAYS: The Discovery and the mature organism but continues in certain tissues throughout Characterization of MPF life. Millions of cells residing within the marrow of your bones or the lining of your intestinal tract are undergoing division at this very moment. This enormous output of cells is needed to replace cells that have aged or died. Although cell division occurs in all organisms, it takes place very differently in prokaryotes and eukaryotes. We will restrict discussion to the eukaryotic version. Two distinct types of eukaryotic cell division will be discussed in this chapter. Mitosis leads to production of cells that are genetically identical to their parent, whereas meiosis leads to production of cells with half the genetic content of the parent. Mitosis serves as the basis for producing new cells, meiosis as the basis for producing new Fluorescence micrograph of a mitotic spindle that had assembled in a cell-free extract prepared from frog eggs, which are cells that lack a centrosome. The red spheres consist of chromatin-covered beads that were added to the extract. -

Cell Life Cycle and Reproduction the Cell Cycle (Cell-Division Cycle), Is a Series of Events That Take Place in a Cell Leading to Its Division and Duplication
Cell Life Cycle and Reproduction The cell cycle (cell-division cycle), is a series of events that take place in a cell leading to its division and duplication. The main phases of the cell cycle are interphase, nuclear division, and cytokinesis. Cell division produces two daughter cells. In cells without a nucleus (prokaryotic), the cell cycle occurs via binary fission. Interphase Gap1(G1)- Cells increase in size. The G1checkpointcontrol mechanism ensures that everything is ready for DNA synthesis. Synthesis(S)- DNA replication occurs during this phase. DNA Replication The process in which DNA makes a duplicate copy of itself. Semiconservative Replication The process in which the DNA molecule uncoils and separates into two strands. Each original strand becomes a template on which a new strand is constructed, resulting in two DNA molecules identical to the original DNA molecule. Gap 2(G2)- The cell continues to grow. The G2checkpointcontrol mechanism ensures that everything is ready to enter the M (mitosis) phase and divide. Mitotic(M) refers to the division of the nucleus. Cell growth stops at this stage and cellular energy is focused on the orderly division into daughter cells. A checkpoint in the middle of mitosis (Metaphase Checkpoint) ensures that the cell is ready to complete cell division. The final event is cytokinesis, in which the cytoplasm divides and the single parent cell splits into two daughter cells. Reproduction Cellular reproduction is a process by which cells duplicate their contents and then divide to yield multiple cells with similar, if not duplicate, contents. Mitosis Mitosis- nuclear division resulting in the production of two somatic cells having the same genetic complement (genetically identical) as the original cell. -

Fishery Science – Biology & Ecology
Fishery Science – Biology & Ecology How Fish Reproduce Illustration of a generic fish life cycle. Source: Zebrafish Information Server, University of South Carolina (http://zebra.sc.edu/smell/nitin/nitin.html) Reproduction is an essential component of life, and there are a diverse number of reproductive strategies in fishes throughout the world. In marine fishes, there are three basic reproductive strategies that can be used to classify fish. The most common reproductive strategy in marine ecosystems is oviparity. Approximately 90% of bony and 43% of cartilaginous fish are oviparous (See Types of Fish). In oviparous fish, females spawn eggs into the water column, which are then fertilized by males. For most oviparous fish, the eggs take less energy to produce so the females release large quantities of eggs. For example, a female Ocean Sunfish is able to produce 300 million eggs over a spawning cycle. The eggs that become fertilized in oviparous fish may spend long periods of time in the water column as larvae before settling out as juveniles. An advantage of oviparity is the number of eggs produced, because it is likely some of the offspring will survive. However, the offspring are at a disadvantage because they must go through a larval stage in which their location is directed by oceans currents. During the larval stage, the larvae act as primary consumers (See How Fish Eat) in the food web where they must not only obtain food but also avoid predation. Another disadvantage is that the larvae might not find suitable habitat when they settle out of the ~ Voices of the Bay ~ [email protected] ~ http://sanctuaries.noaa.gov/education/voicesofthebay.html ~ (Nov 2011) Fishery Science – Biology & Ecology water column. -
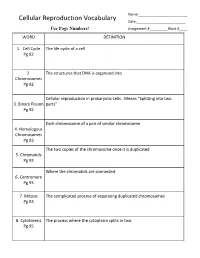
Cellular Reproduction Vocabulary Date:______Use Page Numbers! Assignment #______Block #____ WORD DEFINITION
Name:_________________________ Cellular Reproduction Vocabulary Date:_________________________ Use Page Numbers! Assignment #_________ Block #____ WORD DEFINITION 1. Cell Cycle The life cycle of a cell Pg 92 2. The structures that DNA is organized into Chromosomes Pg 92 Cellular reproduction in prokaryotic cells. Means “Splitting into two 3. Binary Fission parts” Pg 92 Each chromosome of a pair of similar chromosome 4. Homologous Chromosomes Pg 93 The two copies of the chromosome once it is duplicated 5. Chromatids Pg 93 Where the chromatids are connected 6. Centromere Pg 93 7. Mitosis The complicated process of separating duplicated chromosomes Pg 93 8. Cytokinesis The process where the cytoplasm splits in two Pg 95 WORD DEFINITION 9. Budding A type of asexual reproduction, where a piece of the parents body Pg 612 develops into an independent organism 10. A type of asexual reproduction, where the organism breaks into two or Regeneration more parts, each growing into a new organism that is genetically identical (fragmentation) to the parent Pg 612 The production of offspring by combining the genetic material of more 11. Sexual than one parent reproduction Pg 613 A single parent has offspring that are identical to itself 12. Asexual reproduction Pg 613 The male sex cell 13. Sperm Pg 613 The female sex cell 14. Egg Pg 613 The new type of cell that is made when an egg’s nucleus fuses with a 15. Zygote sperm’s nucleus Pg 613 16. Spores Small reproductive cells that are protected by a thick cell wall Pg 256 WORD DEFINITION 17. Sex Cells Specialized cells that combine to form a zygote, they have half the normal Pg 114 number of chromosomes, one of each pair. -

Reproduction in Plants and Animals
Reproduction in Plants and Animals Imagine a gardener checking on his growing plants at the beginning of spring. He notices a few tiny insects eating some of his plants. The gardener isn’t worried—a few insects are not a concern. But when he comes back several weeks later, his plants are covered in these small insects. There are at least ten times as many insects as there were several weeks ago! Where did all of these insects come from? How do organisms make more of their species? Reproduction produces offspring Reproduction is a process by which an organism produces offspring, or young. All organisms reproduce. If they didn’t, no species would survive past a single generation. The tiny insects developing Reproduction allows organisms to pass on their traits, or inside these eggs will grow characteristics to their offspring. Parents pass on their into adult insects. traits through their genetic material, or DNA. Sexual Reproduction requires two parents Sexual reproduction requires a male and female. Each parent contributes half of their genetic material, or DNA, to their offspring. The female contributes her DNA in an egg cell. The male contributes his DNA in a sperm cell. When the egg and sperm combine, they form the new offspring. Offspring may look similar to their parents, but they are not exact copies. In sexual reproduction, each offspring has a mixture of its parent’s traits. Parents may pass on dominant traits or recessive traits to their offspring. Each offspring may be different from its siblings. For These puppies are a product example, suppose the father in a human family does not of sexual reproduction have freckles, but his wife does. -

Project Quest Model Life Science Lessons Genetics Set
Project QuEST Model Life Science Lessons Genetics Set These materials were produced by CREATE with funding from the U.S Department of Education (ED), Institute of Education Sciences, under Contract No. ED-R305A05056. The opinions expressed herein do not necessarily reflect the positions or policies of ED. © 2012 Center for Applied Linguistics www.cal.org/create Project QuEST Model Life Science Lesson Teacher Guide: Asexual and Sexual Reproduction These materials were produced by CREATE with funding from the U.S Department of Education (ED), Institute of Education Sciences, under Contract No. ED-R305A05056. The opinions expressed herein do not necessarily reflect the positions or policies of ED. © 2012 Center for Applied Linguistics www.cal.org/create CREATE Model Life Science Lesson Genetics Set: Asexual and Sexual Reproduction Teacher Guide Middle School Science: Life Science Genetics Set: Asexual and Sexual Reproduction Framework for K-12 Science Education: Dimension 3—Life Science • Disciplinary Core Idea (LS1.B)—Growth and Development of Organisms: Organisms reproduce, either sexually or asexually, and transfer their genetic information to their offspring. • Science and Engineering Practices: Developing and Using Models • Crosscutting Concepts: Cause and Effect Connections to the Common Core State Standards (ELA) • RST.3: Follow precisely a multistep procedure when carrying out experiments, taking measurements, or performing technical tasks. • L6: Acquire and use accurately grade-appropriate general academic and domain- specific words and phrases; gather vocabulary knowledge when considering a word or phrase important to comprehension or expression. Connection to the Common Core State Standards (Math) • MP.2: Reason abstractly and quantitatively. Connections to English Language Development Standards1 • ELD Standard 4: Language of Science o Reading: Explain how organisms reproduce asexually by matching an illustration of their reproduction to the description. -

Living Environment Vocabulary by Prentice Hall 2001 Review Book Unit
Living Environment Vocabulary By Prentice Hall 2001 Review Book Unit Similarities and Topic 1 Differences Among Living Organisms cell the basic unit of structure and function that makes up all organisms metabolism all the chemical reactions that occur within the cells of an organism homeostasis the ability of an organism to maintain a stable internal environment even when the external environment changes reproduction the process by which organisms produce new organisms of the same type cell respiration the process in which nutrients are broken apart, releasing the chemical energy stored in them synthesis a life process that involves combining simple substances into more complex substances organic term used to describe molecules that contain both hydrogen and carbon inorganic a type of molecule that does not contain both carbon and hydrogen but can contain any other combination of elements organelle a structure within the cell that carries out a specific function tissues a group of specialized cells that perform a specific function organ a body structure made of different kinds of tissues combined to perform a specific function organ system several organs that work together to perform a major function in the body cytoplasm the jellylike substance that is between the cell membrane and the nucleus and that contains specialized structures nucleus a large structure within a cell that controls the cell’s metabolism and stores genetic information, including chromosomes and DNA vacuoles storage sacs within the cytoplasm of a cell that may contain -

Cell Theory Tutorial TEK 7.12F: Recognize That According to Cell
Name: ___________________________________________ Cell Theory Tutorial TEK 7.12F: Recognize that according to cell theory all organisms are composed of cells and cells carry on similar functions such as extracting energy from food to sustain life. • A cell is the basic unit of function and structure in all living organisms. There are many species of single-cell organisms than can carry out all of the functions necessary to life within their single cell. • The first scientist to see a “cell” through a microscope was Robert Hooke in the year 1663. He thought that the rectangular compartments that he saw in the bark of the cork oak tree looked like the small rooms or cells in which the monks of a monastery lived. Today we also refer to small rooms in prisons or jails as cells. • Only plant cells are rectangular, but we still use the term “cell” to refer to the smallest self-contained unit of any living organism. • At about the same time as Hooke first observed plant cells, Anton van Leewenhoek observed bacteria, single-cell organisms we now call protists, and small multi- cellular organisms such as hydras. In the next hundred years, the quality of microscopes improved and many scientists studied all life forms to better understand their detailed structures. • In 1838, German scientist Matthias Schleiden concluded that all plants are made of cells. The next year Theodor Schwann, another German, concluded that all animals were also made of cells. • The last piece of the cell theory puzzle came in 1855, when Rudolf Virchow concluded that all cells formed from existing cells. -

Cell Theory 1 Cell Theory
Cell theory 1 Cell theory Cell theory refers to the idea that cells are the basic unit of structure in every living thing. Development of this theory during the mid 17th century was made possible by advances in microscopy. This theory is one of the foundations of biology. The theory says that new cells are formed from other existing cells, and that the cell is a fundamental unit of structure, function and organization in all living organisms. A prokaryote Cell theory 2 History The cell was discovered by Robert Hooke in 1665. He examined (under a coarse, compound microscope) very thin slices of cork and saw a multitude of tiny pores that he remarked looked like the walled compartments of a honeycomb. Because of this association, Hooke called them cells, the name they still bear. However, Hooke did not know their real structure or function.[1] Hooke's description of these cells (which were actually non-living cell walls) was published in Micrographia.[2] . His cell observations gave no indication of the nucleus and other organelles found in most living cells. The first man to witness a live cell under a microscope was Antony van Leeuwenhoek (although the first man to make a compound microscope was Zacharias Janssen), who in 1674 described the algae Spirogyra and named the moving organisms animalcules, meaning "little animals".[3] . Leeuwenhoek probably also saw bacteria.[4] Cell theory was in contrast to the vitalism theories proposed before the discovery of cells. The idea that cells were separable into individual units Drawing of the structure of cork by Robert Hooke that appeared in was proposed by Ludolph Christian Treviranus[5] and Micrographia Johann Jacob Paul Moldenhawer[6] . -

Plants of the World Educator Guide
Educator Guide Presented by The Field Museum Education Department INSIDE: s)NTRODUCTIONTO"OTANY s0LANT$IVISIONSAND2ELATED2ESEARCH s#URRICULUM#ONNECTIONS s&OCUSED&IELD4RIP!CTIVITIES s+EY4ERMS The production of this Educator Guide was supported by the National Science Foundation (grants DEB-1025861 and DEB-1145898 to The Field Museum). Introduction to Botany Botany is the scientific study of plants and fungi. Botanists (plant scientists) in The Field Museum’s Science and Education Department are interested in learning why there are so many different plants and fungi in the world, how this diversity is distributed across the globe, how best to classify it, and what important roles these organisms play in the environment and in human cultures. The Field Museum acquired its first botanical collections from the World’s Columbian Exposition of 1893 when Charles F. Millspaugh, a physician and avid botanist, began soliciting donations of exhibited collections for the Museum. In 1894, the Museum’s herbarium was established with Millspaugh as the Museum’s first Curator of Botany. He helped to expand the herbarium to 50,000 specimens by 1898, and his early work set the stage for the Museum’s long history of botanical exploration. Today, over 70 major botanical expeditions have established The Field’s herbarium as one of the world’s preeminent repositories of plants and fungi with more than 2.7 million specimens. These collections are used to study biodiversity, evolution, conservation, and ecology, and also serves as a depository for important specimens and material used for drug screening. While flowering plants have been a major focus during much of the department’s history, more recently several staff have distinguished themselves in the areas of economic botany, evolutionary biology and mycology.