Synaptic Reshaping and Neuronal Outcomes in the Temporal Lobe Epilepsy
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Thalamohippocampal Atrophy in Focal Epilepsy of Unknown Cause at the Time of Diagnosis
medRxiv preprint doi: https://doi.org/10.1101/2020.07.24.20159368; this version posted July 25, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-ND 4.0 International license . Thalamohippocampal atrophy in focal epilepsy of unknown cause at the time of diagnosis Nicola J. Leek1, Barbara A. K. Kreilkamp1,2, Mollie Neason1, Christophe de Bezenac1, Besa Ziso2, Samia Elkommos3, Kumar Das2, Anthony G. Marson1,2, Simon S. Keller1,2* 1Department of Pharmacology and Therapeutics, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, UK 2The Walton Centre NHS Foundation Trust, Liverpool, UK 3St George’s University Hospitals NHS Foundation Trust, London, UK *Corresponding author Dr Simon Keller Clinical Sciences Centre Aintree University Hospital Lower Lane Liverpool L9 7LJ [email protected] Keywords: Newly diagnosed epilepsy, focal epilepsy, thalamus, hippocampus, treatment outcome NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. 1 medRxiv preprint doi: https://doi.org/10.1101/2020.07.24.20159368; this version posted July 25, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-ND 4.0 International license . Abstract Background: Patients with chronic focal epilepsy may have atrophy of brain structures important for the generation and maintenance of seizures. -

A Guide to Glutamate Receptors
A guide to glutamate receptors 1 Contents Glutamate receptors . 4 Ionotropic glutamate receptors . 4 - Structure ........................................................................................................... 4 - Function ............................................................................................................ 5 - AMPA receptors ................................................................................................. 6 - NMDA receptors ................................................................................................. 6 - Kainate receptors ............................................................................................... 6 Metabotropic glutamate receptors . 8 - Structure ........................................................................................................... 8 - Function ............................................................................................................ 9 - Group I: mGlu1 and mGlu5. .9 - Group II: mGlu2 and mGlu3 ................................................................................. 10 - Group III: mGlu4, mGlu6, mGlu7 and mGlu8 ............................................................ 10 Protocols and webinars . 11 - Protocols ......................................................................................................... 11 - Webinars ......................................................................................................... 12 References and further reading . 13 Excitatory synapse pathway -

Glycine Activated Ion Channel Subunits Encoded by Ctenophore
Glycine activated ion channel subunits encoded by PNAS PLUS ctenophore glutamate receptor genes Robert Albersteina, Richard Greya, Austin Zimmeta, David K. Simmonsb, and Mark L. Mayera,1 aLaboratory of Cellular and Molecular Neurophysiology, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892; and bThe Whitney Laboratory for Marine Bioscience, University of Florida, St. Augustine, FL 32080 Edited by Christopher Miller, Howard Hughes Medical Institute, Brandeis University, Waltham, MA, and approved September 2, 2015 (received for review July 13, 2015) Recent genome projects for ctenophores have revealed the subunits and glutamate to GluN2 subunits for activation of ion presence of numerous ionotropic glutamate receptors (iGluRs) in channel gating (12, 14–17), as well as depolarization to relieve + Mnemiopsis leidyi and Pleurobrachia bachei, among our earliest ion channel block by extracellular Mg2 (18, 19). The initial metazoan ancestors. Sequence alignments and phylogenetic analy- annotation of the M. leidyi genome identified 16 candidate iGluR sis show that these form a distinct clade from the well-characterized genes (4), whereas in the draft genome of P. bachei, 14 iGluRs AMPA, kainate, and NMDA iGluR subtypes found in vertebrates. were annotated as kainate-like receptors (5). In view of growing Although annotated as glutamate and kainate receptors, crystal interest in the molecular evolution of ion channels and receptors, structures of the ML032222a and PbiGluR3 ligand-binding domains and the pivotal role that ctenophores play in our current un- (LBDs) reveal endogenous glycine in the binding pocket, whereas derstanding of nervous system development (20), we initiated a ligand-binding assays show that glycine binds with nanomolar af- structural and functional characterization of glutamate receptors finity; biochemical assays and structural analysis establish that glu- expressed in both species. -

Reversible Signal Abnormalities in the Hippocampus and Neocortex After Prolonged Seizures
Reversible Signal Abnormalities in the Hippocampus and Neocortex after Prolonged Seizures Stephen Chan, Steven S. M. Chin, Krishnan Kartha, Douglas R. Nordli, Robert R. Goodman, Timothy A. Pedley, and Sadek K. Hilal PURPOSE: To investigate the phenomenon of reversible increased signal intensity of medial temporal lobe structures and cerebral neocortex seen on MR images of six patients with recent prolonged seizure activity. METHODS: After excluding patients with known causes of reversible signal abnormalities (such as hypertensive encephalopathy), we retrospectively reviewed the clinical findings and MR studies of six patients whose MR studies showed reversible signal abnor- malities. MR pulse sequences included T2-weighted spin-echo coronal views or conventional short-tau inversion-recovery coronal images of the temporal lobes. RESULTS: All six MR studies showed increased signal intensity within the medial temporal lobe, including the hippocampus in five studies. All follow-up MR examinations showed partial or complete resolution of the hyperin- tensity within the medial temporal lobe and the neocortex. In one patient, results of a brain biopsy revealed severe cerebral cortical gliosis. Temporal lobectomy performed 4 years later showed moderate cortical gliosis and nonspecific hippocampal cell loss and gliosis. CONCLUSION: Sig- nificant hyperintensity within the temporal lobe is demonstrable on MR images after prolonged seizure activity, suggestive of seizure-induced edema or gliosis. Damage to medial temporal lobe structures by prolonged seizure activity indicates a possible mechanism of epileptogenic disorders. Index terms: Brain, magnetic resonance; Brain, temporal lobe; Hippocampus; Seizures AJNR Am J Neuroradiol 17:1725–1731, October 1996 Prolonged seizure activity is associated with character of the acute neuronal loss in the hip- long-lasting neurologic damage and even death pocampus seen after an episode of status epi- in humans (1–3); prompt treatment is required lepticus is different from the neuronal loss and to forestall irreversible changes (4). -

Interplay Between Gating and Block of Ligand-Gated Ion Channels
brain sciences Review Interplay between Gating and Block of Ligand-Gated Ion Channels Matthew B. Phillips 1,2, Aparna Nigam 1 and Jon W. Johnson 1,2,* 1 Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA 15260, USA; [email protected] (M.B.P.); [email protected] (A.N.) 2 Center for Neuroscience, University of Pittsburgh, Pittsburgh, PA 15260, USA * Correspondence: [email protected]; Tel.: +1-(412)-624-4295 Received: 27 October 2020; Accepted: 26 November 2020; Published: 1 December 2020 Abstract: Drugs that inhibit ion channel function by binding in the channel and preventing current flow, known as channel blockers, can be used as powerful tools for analysis of channel properties. Channel blockers are used to probe both the sophisticated structure and basic biophysical properties of ion channels. Gating, the mechanism that controls the opening and closing of ion channels, can be profoundly influenced by channel blocking drugs. Channel block and gating are reciprocally connected; gating controls access of channel blockers to their binding sites, and channel-blocking drugs can have profound and diverse effects on the rates of gating transitions and on the stability of channel open and closed states. This review synthesizes knowledge of the inherent intertwining of block and gating of excitatory ligand-gated ion channels, with a focus on the utility of channel blockers as analytic probes of ionotropic glutamate receptor channel function. Keywords: ligand-gated ion channel; channel block; channel gating; nicotinic acetylcholine receptor; ionotropic glutamate receptor; AMPA receptor; kainate receptor; NMDA receptor 1. Introduction Neuronal information processing depends on the distribution and properties of the ion channels found in neuronal membranes. -

Cellular Trafficking of Nicotinic Acetylcholine Receptors
npg Acta Pharmacol Sin 2009 Jun; 30 (6): 656–662 Review Cellular trafficking of nicotinic acetylcholine receptors Paul A ST JOHN* Department of Cell Biology and Anatomy, University of Arizona College of Medicine, Tucson, AZ 85724, USA Nicotinic acetylcholine receptors (nAChRs) play critical roles throughout the body. Precise regulation of the cellular loca- tion and availability of nAChRs on neurons and target cells is critical to their proper function. Dynamic, post-translational regulation of nAChRs, particularly control of their movements among the different compartments of cells, is an important aspect of that regulation. A combination of new information and new techniques has the study of nAChR trafficking poised for new breakthroughs. Keywords: membrane traffic; protein traffic; biosynthesis; endocytosis; endoplasmic reticulum-associated degradation Acta Pharmacologica Sinica (2009) 30: 656–662; doi: 10.1038/aps.2009.76 Introduction ways, but two particular perturbations have been especially well studied and exert their effects at least in part by altering Nicotinic acetylcholine receptors (nAChRs) mediate the trafficking of nAChRs: 1) denervation changes the total synaptic transmission in the CNS, in autonomic ganglia, and number, the distribution, and the turnover rate of nAChRs in at neuromuscular junctions and other peripheral synapses. skeletal muscle; 2) prolonged exposure to nicotine increases The functional properties of these synapses differ, but in each the total number of nAChRs in neurons. Several of the stud- case, properly functional signaling requires cellular control ies reviewed here addressed the mechanisms by which these of the number, type, and location of nAChRs. Trafficking treatments alter nAChR trafficking. Other authors in this of nAChRs – the movement of nAChRs between compart- special issue will address other aspects of the effects of nico- ments of a cell, including the cell's biosynthetic and degrada- tine on nAChRs. -

Kainate Receptors Depress Excitatory Synaptic Transmission at CA33CA1 Synapses in the Hippocampus Via a Direct Presynaptic Action
The Journal of Neuroscience, May 1, 2001, 21(9):2958–2966 Kainate Receptors Depress Excitatory Synaptic Transmission at CA33CA1 Synapses in the Hippocampus via a Direct Presynaptic Action Matthew Frerking,1 Dietmar Schmitz,1 Qiang Zhou,1 Joshua Johansen,2 and Roger A. Nicoll1,2 Departments of 1Cellular and Molecular Pharmacology and 2Physiology, University of California, San Francisco, California 94143-0450 Kainate receptor activation depresses synaptic release of neu- excitation and subsequent release of a neuromodulator. Pre- rotransmitter at a number of synapses in the CNS. The mech- synaptic depolarization, achieved via increasing extracellular anism underlying this depression is controversial, and both K ϩ, caused a depression of the presynaptic fiber volley and an ionotropic and metabotropic mechanisms have been sug- increase in the frequency of miniature EPSCs. Neither effect gested. We report here that the AMPA/kainate receptor ago- was observed with DA, suggesting that DA does not depress nists domoate (DA) and kainate (KA) cause a presynaptic de- transmission via a presynaptic depolarization. However, the pression of glutamatergic transmission at CA33CA1 synapses effects of DA were abolished by the G-protein inhibitors in the hippocampus, which is not blocked by the AMPA recep- N-ethylmaleimide and pertussis toxin. These results suggest tor antagonist GYKI 53655 but is blocked by the AMPA/KA that KA receptor activation depresses synaptic transmission at receptor antagonist CNQX. Neither a blockade of interneuronal this synapse via a direct, presynaptic, metabotropic action. discharge nor antagonists of several neuromodulators affect Key words: domoate; kainate; metabotropic; presynaptic; the depression, suggesting that it is not the result of indirect hippocampus; CA1 Neurotransmitter receptors in the CNS can be separated into two use-dependent depression. -

Infection on Neurological Implanted Devices
ECCMID Amsterdam 09.04.2016 Challenging complex infections for ID physicians Infection on neurological implanted devices Anna Conen, MD MSc Deputy Head Physician Division of Infectious Diseases and Hospital Hygiene ESCMIDKantonsspital Aarau, eLibrary Switzerland by author Disclosures Received travel grants from Gilead, Merck Sharp Dohme, ViiV Healthcare, Bristol- Myers Squibb and Janssen. ESCMID eLibrary by author Outline • Diagnosis of implant-associated infections • Treatment concepts of implant-associated infections • Specific infections associated with the following implants: Craniotomy/bone flap Cranioplasty Deep brain stimulator Ventriculo-peritoneal shunt Neurological implants ESCMIDSpinal cord stimulator External ventricular eLibrary drainage Ventriculo-atrial shunt by author Risk of implant-associated infections Device No. inserted in the US, Infection rate, % per year Fracture fixation devices 2,000,000 5–10 Dental implants 1,000,000 5–10 Joint prostheses 600,000 1–3 Neurosurgical implants 450,000 3–15 Cardiac pacemakers 300,000 1–7 Mammary implants 130,000 1–2 Mechanical heart valves 85,000 1–3 Penile implants 15,000 1–3 Heart assist devices 700 25–50 ESCMID eLibraryDarouiche RO. Clin Infect Dis 2011; 33:1567-1572 by author Concept and diagnosis of biofilm Biofilm Sonication - Bacteria adhere to implant - Sonication of implants*: surface detachment of biofilm - Embed in a matrix - Sonication fluid plated on - In stationary growth phase culture media - Slowly replicate Standard method: 3 Sonication of tissue biopsies implant: Sensitivity ~60% Sensitivity 80-90% *Cranioplasty, shunts, screws, plates, stimulators, etc. Zimmerli W. J Infect Dis. 1982;146(4):487-97. Trampuz A. NEJM 2007;357:654–663. Portillo M. J Clin Microbiol 2015;53(5):1622-7. -

Functional Kainate-Selective Glutamate Receptors in Cultured Hippocampal Neurons (Excitatory Amino Acid Receptors/Hippocampus) JUAN LERMA*, ANA V
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 11688-11692, December 1993 Neurobiology Functional kainate-selective glutamate receptors in cultured hippocampal neurons (excitatory amino acid receptors/hippocampus) JUAN LERMA*, ANA V. PATERNAIN, JosE R. NARANJO, AND BRITT MELLSTR6M Departamento de Plasticidad Neural, Instituto Cajal, Consejo Superior de Investigaciones Cientfficas, Avenida Doctor Arce 37, 28002-Madrid, Spain Communicated by Michael V. L. Bennett, September 15, 1993 ABSTRACT Glutamate mediates fast synaptic transmis- experiments, the regional distribution of high-affinity sion at the majority of excitatory synapses throughout the [3H]kainate binding sites does not match the AMPA receptor central nervous system by interacting with different types of distribution but corresponds well to the brain areas with high receptor channels. Cloning of glutamate receptors has pro- susceptibility to the neurotoxic actions of kainate (e.g., vided evidence for the existence of several structurally related hippocampal CA3 field) (13). However, patch-clamp record- subunit families, each composed of several members. It has ings from adult hippocampal neurons have revealed that been proposed that KA1 and KA2 and GluR-5, GluR-6, and native glutamate receptors are similar to the AMPA-type GluR-7 families represent subunit classes of high-affinity kain- recombinant glutamate receptors expressed from cDNA ate receptors and that in vivo different kainate receptor sub- clones but have failed so far to detect receptor channels ofthe types might be constructed from these subunits in heteromeric kainate type (14, 15). The only apparently high-affinity kain- assembly. However, despite some indications from autoradio- ate receptor channels have been found in the peripheral graphic studies and binding data in brain membranes, no nervous system (16, 17), although they are also activated by functional pure kainate receptors have so far been detected in AMPA. -

Microrecording and Image-Guided Stereotactic Biopsy of Deep-Seated Brain Tumors
CLINICAL ARTICLE J Neurosurg 123:978–988, 2015 Microrecording and image-guided stereotactic biopsy of deep-seated brain tumors Keiya Iijima, MD,1 Masafumi Hirato, MD, PhD,1 Takaaki Miyagishima, MD, PhD,1 Keishi Horiguchi, MD, PhD,1 Kenichi Sugawara, MD, PhD,1 Junko Hirato, MD, PhD,3 Hideaki Yokoo, MD, PhD,2 and Yuhei Yoshimoto, MD, PhD1 Departments of 1Neurosurgery and 2Human Pathology, Gunma University Graduate School of Medicine; and 3Clinical Department of Pathology, Gunma University Hospital, Maebashi, Gunma, Japan OBJECT Image-guided stereotactic brain tumor biopsy cannot easily obtain samples of small deep-seated tumor or se- lectively sample the most viable region of malignant tumor. Image-guided stereotactic biopsy in combination with depth microrecording was evaluated to solve such problems. METHODS Operative records, MRI findings, and pathological specimens were evaluated in 12 patients with small deep-seated brain tumor, in which image-guided stereotactic biopsy was performed with the aid of depth microrecording. The tumors were located in the caudate nucleus (1 patient), thalamus (7 patients), midbrain (2 patients), and cortex (2 patients). Surgery was performed with a frameless stereotactic system in 3 patients and with a frame-based stereotactic system in 9 patients. Microrecording was performed to study the electrical activities along the trajectory in the deep brain structures and the tumor. The correlations were studied between the electrophysiological, MRI, and pathological find- ings. Thirty-two patients with surface or large brain tumor were also studied, in whom image-guided stereotactic biopsy without microrecording was performed. RESULTS The diagnostic yield in the group with microrecording was 100% (low-grade glioma 4, high-grade glioma 4, diffuse large B-cell lymphoma 3, and germinoma 1), which was comparable to 93.8% in the group without microrecord- ing. -
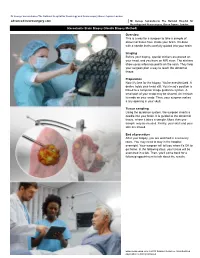
Advanced-Neurosurgery.Com Stereotactic
Dr George Samandouras The National Hospital for Neurology and Neurosurgery Queen Square London advanced-neurosurgery.com Mr George Samandouras The National Hospital for Neurology and Neurosurgery, Queen Square, London Stereotactic Brain Biopsy (Needle Biopsy Method) Overview This is a way for a surgeon to take a sample of abnormal tissue from inside your brain. It's done with a needle that's carefully guided into your brain. Imaging Before your biopsy, special stickers are placed on your head, and you have an MRI scan. The stickers show up as reference points on the scan. They help your surgeon plan a way to reach the abnormal tissue. Preparation Now it's time for the biopsy. You're anesthetized. A device holds your head still. Your head's position is linked to a computer image guidance system. A small part of your scalp may be shaved. An incision is made on your scalp. Then, your surgeon makes a tiny opening in your skull. Tissue sampling Using the guidance system, the surgeon inserts a needle into your brain. It is guided to the abnormal tissue, where it takes a sample. More than one sample may be needed. Finally, your skull and your skin are closed. End of procedure After your biopsy, you are watched in a recovery room. You may need to stay in the hospital overnight. Your surgeon will tell you when it's OK to go home. In the following days, your tissue will be examined in a lab. Then, you'll come back for a followup appointment to talk about the results. -

Surgical Management of Brain Tumors
SURGICAL MANAGEMENT OF BRAIN TUMORS JASSIN M. JOURIA, MD DR. JASSIN M. JOURIA IS A MEDICAL DOCTOR, PROFESSOR OF ACADEMIC MEDICINE, AND MEDICAL AUTHOR. HE GRADUATED FROM ROSS UNIVERSITY SCHOOL OF MEDICINE AND HAS COMPLETED HIS CLINICAL CLERKSHIP TRAINING IN VARIOUS TEACHING HOSPITALS THROUGHOUT NEW YORK, INCLUDING KING’S COUNTY HOSPITAL CENTER AND BROOKDALE MEDICAL CENTER, AMONG OTHERS. DR. JOURIA HAS PASSED ALL USMLE MEDICAL BOARD EXAMS, AND HAS SERVED AS A TEST PREP TUTOR AND INSTRUCTOR FOR KAPLAN. HE HAS DEVELOPED SEVERAL MEDICAL COURSES AND CURRICULA FOR A VARIETY OF EDUCATIONAL INSTITUTIONS. DR. JOURIA HAS ALSO SERVED ON MULTIPLE LEVELS IN THE ACADEMIC FIELD INCLUDING FACULTY MEMBER AND DEPARTMENT CHAIR. DR. JOURIA CONTINUES TO SERVES AS A SUBJECT MATTER EXPERT FOR SEVERAL CONTINUING EDUCATION ORGANIZATIONS COVERING MULTIPLE BASIC MEDICAL SCIENCES. HE HAS ALSO DEVELOPED SEVERAL CONTINUING MEDICAL EDUCATION COURSES COVERING VARIOUS TOPICS IN CLINICAL MEDICINE. RECENTLY, DR. JOURIA HAS BEEN CONTRACTED BY THE UNIVERSITY OF MIAMI/JACKSON MEMORIAL HOSPITAL’S DEPARTMENT OF SURGERY TO DEVELOP AN E- MODULE TRAINING SERIES FOR TRAUMA PATIENT MANAGEMENT. DR. JOURIA IS CURRENTLY AUTHORING AN ACADEMIC TEXTBOOK ON HUMAN ANATOMY & PHYSIOLOGY. Abstract The field of brain tumor research, diagnosis, and treatment is rapidly evolving. Over 120 types of brain tumors have been identified to date, and that number continues to increase. As the information available about brain tumors grows, so does the ability to target screening and therapies to provide patients with optimal outcomes. It is critical that health clinicians understand the surgical and treatment options in order to educate patients and to develop a care plan that has a positive outcome while respecting the patient's needs and desires.