Surgical Management of Brain Tumors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genetic Markers in Lung Cancer Diagnosis: a Review
International Journal of Molecular Sciences Review Genetic Markers in Lung Cancer Diagnosis: A Review Katarzyna Wadowska 1 , Iwona Bil-Lula 1 , Łukasz Trembecki 2,3 and Mariola Sliwi´ ´nska-Mosso´n 1,* 1 Department of Medical Laboratory Diagnostics, Division of Clinical Chemistry and Laboratory Haematology, Wroclaw Medical University, 50-556 Wroclaw, Poland; [email protected] (K.W.); [email protected] (I.B.-L.) 2 Department of Radiation Oncology, Lower Silesian Oncology Center, 53-413 Wroclaw, Poland; [email protected] 3 Department of Oncology, Faculty of Medicine, Wroclaw Medical University, 53-413 Wroclaw, Poland * Correspondence: [email protected]; Tel.: +48-71-784-06-30 Received: 1 June 2020; Accepted: 25 June 2020; Published: 27 June 2020 Abstract: Lung cancer is the most often diagnosed cancer in the world and the most frequent cause of cancer death. The prognosis for lung cancer is relatively poor and 75% of patients are diagnosed at its advanced stage. The currently used diagnostic tools are not sensitive enough and do not enable diagnosis at the early stage of the disease. Therefore, searching for new methods of early and accurate diagnosis of lung cancer is crucial for its effective treatment. Lung cancer is the result of multistage carcinogenesis with gradually increasing genetic and epigenetic changes. Screening for the characteristic genetic markers could enable the diagnosis of lung cancer at its early stage. The aim of this review was the summarization of both the preclinical and clinical approaches in the genetic diagnostics of lung cancer. The advancement of molecular strategies and analytic platforms makes it possible to analyze the genome changes leading to cancer development—i.e., the potential biomarkers of lung cancer. -

DCIS): Pathological Features, Differential Diagnosis, Prognostic Factors and Specimen Evaluation
Modern Pathology (2010) 23, S8–S13 S8 & 2010 USCAP, Inc. All rights reserved 0893-3952/10 $32.00 Ductal carcinoma in situ (DCIS): pathological features, differential diagnosis, prognostic factors and specimen evaluation Sarah E Pinder Breast Research Pathology, Research Oncology, Division of Cancer Studies, King’s College London, Guy’s Hospital, London, UK Ductal carcinoma in situ (DCIS) is a heterogeneous, unicentric precursor of invasive breast cancer, which is frequently identified through mammographic breast screening programs. The lesion can cause particular difficulties for specimen handling in the laboratory and typically requires even more diligent macroscopic assessment and sampling than invasive disease. Pitfalls and tips for macroscopic handling, microscopic diagnosis and assessment, including determination of prognostic factors, such as cytonuclear grade, presence or absence of necrosis, size of the lesion and distance to margins are described. All should be routinely included in histopathology reports of this disease; in order not to omit these clinically relevant details, synoptic reports, such as that produced by the College of American Pathologists are recommended. No biomarkers have been convincingly shown, and validated, to predict the behavior of DCIS till date. Modern Pathology (2010) 23, S8–S13; doi:10.1038/modpathol.2010.40 Keywords: ductal carcinoma in situ (DCIS); breast cancer; histopathology; prognostic factors Ductal carcinoma in situ (DCIS) is a malignant, lesions, a good cosmetic result can be obtained by clonal proliferation of cells growing within the wide local excision. Recurrence of DCIS generally basement membrane-bound structures of the breast occurs at the site of previous excision and it is and with no evidence of invasion into surrounding therefore better regarded as residual disease, as stroma. -

Reversible Signal Abnormalities in the Hippocampus and Neocortex After Prolonged Seizures
Reversible Signal Abnormalities in the Hippocampus and Neocortex after Prolonged Seizures Stephen Chan, Steven S. M. Chin, Krishnan Kartha, Douglas R. Nordli, Robert R. Goodman, Timothy A. Pedley, and Sadek K. Hilal PURPOSE: To investigate the phenomenon of reversible increased signal intensity of medial temporal lobe structures and cerebral neocortex seen on MR images of six patients with recent prolonged seizure activity. METHODS: After excluding patients with known causes of reversible signal abnormalities (such as hypertensive encephalopathy), we retrospectively reviewed the clinical findings and MR studies of six patients whose MR studies showed reversible signal abnor- malities. MR pulse sequences included T2-weighted spin-echo coronal views or conventional short-tau inversion-recovery coronal images of the temporal lobes. RESULTS: All six MR studies showed increased signal intensity within the medial temporal lobe, including the hippocampus in five studies. All follow-up MR examinations showed partial or complete resolution of the hyperin- tensity within the medial temporal lobe and the neocortex. In one patient, results of a brain biopsy revealed severe cerebral cortical gliosis. Temporal lobectomy performed 4 years later showed moderate cortical gliosis and nonspecific hippocampal cell loss and gliosis. CONCLUSION: Sig- nificant hyperintensity within the temporal lobe is demonstrable on MR images after prolonged seizure activity, suggestive of seizure-induced edema or gliosis. Damage to medial temporal lobe structures by prolonged seizure activity indicates a possible mechanism of epileptogenic disorders. Index terms: Brain, magnetic resonance; Brain, temporal lobe; Hippocampus; Seizures AJNR Am J Neuroradiol 17:1725–1731, October 1996 Prolonged seizure activity is associated with character of the acute neuronal loss in the hip- long-lasting neurologic damage and even death pocampus seen after an episode of status epi- in humans (1–3); prompt treatment is required lepticus is different from the neuronal loss and to forestall irreversible changes (4). -

Infection on Neurological Implanted Devices
ECCMID Amsterdam 09.04.2016 Challenging complex infections for ID physicians Infection on neurological implanted devices Anna Conen, MD MSc Deputy Head Physician Division of Infectious Diseases and Hospital Hygiene ESCMIDKantonsspital Aarau, eLibrary Switzerland by author Disclosures Received travel grants from Gilead, Merck Sharp Dohme, ViiV Healthcare, Bristol- Myers Squibb and Janssen. ESCMID eLibrary by author Outline • Diagnosis of implant-associated infections • Treatment concepts of implant-associated infections • Specific infections associated with the following implants: Craniotomy/bone flap Cranioplasty Deep brain stimulator Ventriculo-peritoneal shunt Neurological implants ESCMIDSpinal cord stimulator External ventricular eLibrary drainage Ventriculo-atrial shunt by author Risk of implant-associated infections Device No. inserted in the US, Infection rate, % per year Fracture fixation devices 2,000,000 5–10 Dental implants 1,000,000 5–10 Joint prostheses 600,000 1–3 Neurosurgical implants 450,000 3–15 Cardiac pacemakers 300,000 1–7 Mammary implants 130,000 1–2 Mechanical heart valves 85,000 1–3 Penile implants 15,000 1–3 Heart assist devices 700 25–50 ESCMID eLibraryDarouiche RO. Clin Infect Dis 2011; 33:1567-1572 by author Concept and diagnosis of biofilm Biofilm Sonication - Bacteria adhere to implant - Sonication of implants*: surface detachment of biofilm - Embed in a matrix - Sonication fluid plated on - In stationary growth phase culture media - Slowly replicate Standard method: 3 Sonication of tissue biopsies implant: Sensitivity ~60% Sensitivity 80-90% *Cranioplasty, shunts, screws, plates, stimulators, etc. Zimmerli W. J Infect Dis. 1982;146(4):487-97. Trampuz A. NEJM 2007;357:654–663. Portillo M. J Clin Microbiol 2015;53(5):1622-7. -

Understanding Ductal Carcinoma in Situ (DCIS)
Understanding ductal carcinoma in situ (DCIS) and deciding about treatment Understanding ductal carcinoma in situ (DCIS) and deciding about treatment Developed by National Breast and Ovarian Cancer Centre Funded by the Australian Government Department of Health and Ageing Understanding ductal carcinoma in situ Contents Acknowledgements .........................................................................................2 How to use this resource ..............................................................................3 Introduction ...........................................................................................................4 Why do I need treatment for DCIS? .........................................................5 Surgery ......................................................................................................................7 Radiotherapy ......................................................................................................11 What is the risk of developing invasive breast cancer or Understanding ductal carcinoma in situ (DCIS) and deciding about treatment was prepared and produced by: DCIS after treatment? ....................................................................................12 National Breast and Ovarian Cancer Centre What follow-up will I need? .......................................................................17 Level 1 Suite 103/355 Crown Street Surry Hills NSW 2010 How can I get more emotional support? .........................................18 Locked Bag 3 -

Microrecording and Image-Guided Stereotactic Biopsy of Deep-Seated Brain Tumors
CLINICAL ARTICLE J Neurosurg 123:978–988, 2015 Microrecording and image-guided stereotactic biopsy of deep-seated brain tumors Keiya Iijima, MD,1 Masafumi Hirato, MD, PhD,1 Takaaki Miyagishima, MD, PhD,1 Keishi Horiguchi, MD, PhD,1 Kenichi Sugawara, MD, PhD,1 Junko Hirato, MD, PhD,3 Hideaki Yokoo, MD, PhD,2 and Yuhei Yoshimoto, MD, PhD1 Departments of 1Neurosurgery and 2Human Pathology, Gunma University Graduate School of Medicine; and 3Clinical Department of Pathology, Gunma University Hospital, Maebashi, Gunma, Japan OBJECT Image-guided stereotactic brain tumor biopsy cannot easily obtain samples of small deep-seated tumor or se- lectively sample the most viable region of malignant tumor. Image-guided stereotactic biopsy in combination with depth microrecording was evaluated to solve such problems. METHODS Operative records, MRI findings, and pathological specimens were evaluated in 12 patients with small deep-seated brain tumor, in which image-guided stereotactic biopsy was performed with the aid of depth microrecording. The tumors were located in the caudate nucleus (1 patient), thalamus (7 patients), midbrain (2 patients), and cortex (2 patients). Surgery was performed with a frameless stereotactic system in 3 patients and with a frame-based stereotactic system in 9 patients. Microrecording was performed to study the electrical activities along the trajectory in the deep brain structures and the tumor. The correlations were studied between the electrophysiological, MRI, and pathological find- ings. Thirty-two patients with surface or large brain tumor were also studied, in whom image-guided stereotactic biopsy without microrecording was performed. RESULTS The diagnostic yield in the group with microrecording was 100% (low-grade glioma 4, high-grade glioma 4, diffuse large B-cell lymphoma 3, and germinoma 1), which was comparable to 93.8% in the group without microrecord- ing. -
Advanced-Neurosurgery.Com Stereotactic
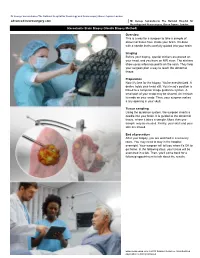
Dr George Samandouras The National Hospital for Neurology and Neurosurgery Queen Square London advanced-neurosurgery.com Mr George Samandouras The National Hospital for Neurology and Neurosurgery, Queen Square, London Stereotactic Brain Biopsy (Needle Biopsy Method) Overview This is a way for a surgeon to take a sample of abnormal tissue from inside your brain. It's done with a needle that's carefully guided into your brain. Imaging Before your biopsy, special stickers are placed on your head, and you have an MRI scan. The stickers show up as reference points on the scan. They help your surgeon plan a way to reach the abnormal tissue. Preparation Now it's time for the biopsy. You're anesthetized. A device holds your head still. Your head's position is linked to a computer image guidance system. A small part of your scalp may be shaved. An incision is made on your scalp. Then, your surgeon makes a tiny opening in your skull. Tissue sampling Using the guidance system, the surgeon inserts a needle into your brain. It is guided to the abnormal tissue, where it takes a sample. More than one sample may be needed. Finally, your skull and your skin are closed. End of procedure After your biopsy, you are watched in a recovery room. You may need to stay in the hospital overnight. Your surgeon will tell you when it's OK to go home. In the following days, your tissue will be examined in a lab. Then, you'll come back for a followup appointment to talk about the results. -

Transmissible Spongiform Encephalopathies (TSE) Including 7.12.18 - Revised Creutzfeldt - Jakob Disease (CJD) 2002 - Author
Section: UTMB On-line Documentation 01.37 - Policy Subject: Infection Control & Healthcare Epidemiology Policies and Procedures Topic: 01.37 - Transmissible Spongiform Encephalopathies (TSE) including 7.12.18 - Revised Creutzfeldt - Jakob Disease (CJD) 2002 - Author 01.37 - Transmissible Spongiform Encephalopathies (TSE) including Creutzfeldt - Jakob Disease (CJD) Purpose To protect healthcare workers from TSE Audience All employees of UTMB hospitals, clinics, contract workers, volunteers, and students At Risk Patients The following patients are considered to be at risk for TSE: Patients with rapidly progressive dementia, ataxia, and myoclonus. Patients who have received cadaver-derived hormones, especially growth hormones before 1970. Healthcare Epidemiology should be notified about all patients considered at high risk for TSE. Table 2 Distribution of Infectivity in the human body1 Infectivity Category Tissues, Secretions, and Excretions High Infectivity Brain Spinal Cord Eye Low Infectivity CSF Kidney Liver Lung Lymph nodes/spleen Placenta No Detectable Infectivity Adipose tissue Adrenal gland Gingival tissue Heart muscle Intestine Peripheral nerve Prostate Skeletal muscle Testis Thyroid gland Tears Nasal mucous Saliva Sweat Serous exudate Milk Semen Urine Feces Blood Page 1 of 9 Section: UTMB On-line Documentation 01.37 - Policy Subject: Infection Control & Healthcare Epidemiology Policies and Procedures Topic: 01.37 - Transmissible Spongiform Encephalopathies (TSE) including 7.12.18 - Revised Creutzfeldt - Jakob Disease (CJD) 2002 - Author 1 Assignment of different organs and tissues to categories of high and low infectivity is chiefly based upon the frequency with which infectivity has been detectable, rather than upon quantitative assays of the level of infectivity, for which data are incomplete. Experimental data include primates inoculated with tissues from human cases of CJD, but have been supplemented in some categories by data obtained from naturally occurring animal TSEs. -

Full Text (PDF)
Published OnlineFirst April 1, 2014; DOI: 10.1158/1940-6207.CAPR-13-0362 Cancer Prevention Research Article Research Inhibition of the Transition of Ductal Carcinoma In Situ to Invasive Ductal Carcinoma by a Gemini Vitamin D Analog Joseph Wahler1, Jae Young So1, Yeoun Chan Kim1, Fang Liu1,2,3, Hubert Maehr1, Milan Uskokovic1, and Nanjoo Suh1,3 Abstract Ductal carcinoma in situ (DCIS) is a nonmalignant lesion of the breast with the potential to progress to invasive ductal carcinoma (IDC). The disappearance and breakdown of the myoepithelial cell layer and basement membrane in DCIS have been identified as major events in the development of breast cancer. The MCF10DCIS.com cell line is a well-established model, which recapitulates the progression of breast cancer from DCIS to IDC. We have previously reported that a novel Gemini vitamin D analog, 1a,25-dihydroxy- 20R-21(3-hydroxy-3-deuteromethyl-4,4,4-trideuterobutyl)-23-yne-26,27-hexafluoro-cholecalciferol (BXL0124) is a potent inhibitor of the growth of MCF10DCIS.com xenografted tumors without hypercal- cemic toxicity. In this study, we utilized the MCF10DCIS.com in vivo model to assess the effects of BXL0124 on breast cancer progression from weeks 1 to 4. Upon DCIS progression to IDC from weeks 3 to 4, tumors lost the myoepithelial cell layer and basement membrane as shown by immunofluorescence staining with smooth muscle actin and laminin 5, respectively. Administration of BXL0124 maintained the critical myoepithelial cell layer as well as basement membrane, and animals treated with BXL0124 showed a 43% reduction in tumor volume by week 4. -

Consensus Guideline on the Management of the Axilla in Patients with Invasive/In-Situ Breast Cancer
- Official Statement - Consensus Guideline on the Management of the Axilla in Patients With Invasive/In-Situ Breast Cancer Purpose To outline the management of the axilla for patients with invasive and in-situ breast cancer. Associated ASBrS Guidelines or Quality Measures 1. Performance and Practice Guidelines for Sentinel Lymph Node Biopsy in Breast Cancer Patients – Revised November 25, 2014 2. Performance and Practice Guidelines for Axillary Lymph Node Dissection in Breast Cancer Patients – Approved November 25, 2014 3. Quality Measure: Sentinel Lymph Node Biopsy for Invasive Breast Cancer – Approved November 4, 2010 4. Prior Position Statement: Management of the Axilla in Patients With Invasive Breast Cancer – Approved August 31, 2011 Methods A literature review inclusive of recent randomized controlled trials evaluating the use of sentinel lymph node surgery and axillary lymph node dissection for invasive and in-situ breast cancer as well as the pathologic review of sentinel lymph nodes and indications for axillary radiation was performed. This is not a complete systematic review but rather, a comprehensive review of recent relevant literature. A focused review of non-randomized controlled trials was then performed to develop consensus guidance on management of the axilla in scenarios where randomized controlled trials data is lacking. The ASBrS Research Committee developed a consensus document, which was reviewed and approved by the ASBrS Board of Directors. Summary of Data Reviewed Recommendations Based on Randomized Controlled -

Techniques for the Application of Stereotactic Head Frames Based on a 25-Year Experience
Open Access Technical Report DOI: 10.7759/cureus.543 Techniques for the Application of Stereotactic Head Frames Based on a 25-Year Experience Michael Safaee 1 , John Burke 1 , Michael W. McDermott 1 1. Department of Neurological Surgery, University of California, San Francisco Corresponding author: Michael Safaee, [email protected] Abstract The use of skull fixed stereotactic head frames remains an integral part of neurosurgical practice. Methods for the positioning, anesthesia, and fixation have been described in various publications. The authors describe the steps used currently that reflect a 25-year experience with stereotactic frame application. Photographs were obtained throughout the set-up and frame application process. The step-by-step methods were described with accompanying figures. Consent was obtained from that patient to allow for photographs throughout the frame application process. Consent was also obtained from a separate patient for videotaping the entire application process. Descriptive tags are embedded in the video to assist with the instruction of the senior author's (MWM) methods. The senior author has used the described method in over 1,000 cases. A recent analysis of the patient pain experience has been reported and is well tolerated. Supplemental devices beyond the manufacturers' standard equipment have been employed or developed: ethyl chloride spray, angled front posts, frame positioner, and torque wrenches. There have been no shunt perforations, no cranial vault penetrations, one titanium mesh cranioplasty deformation, three pin site infections (3 patients; 4,000 pin sites; 0.075%), and one thermal injury (0.025%). Stereotactic head frame application remains an important part of neurosurgical practice. -

EAU Guidelines on the Diagnosis and Treatment of Urothelial Carcinoma in Situ Adrian P.M
European Urology European Urology 48 (2005) 363–371 EAU Guidelines EAU Guidelines on the Diagnosis and Treatment of Urothelial Carcinoma in Situ Adrian P.M. van der Meijdena,*, Richard Sylvesterb, Willem Oosterlinckc, Eduardo Solsonad, Andreas Boehlee, Bernard Lobelf, Erkki Rintalag for the EAU Working Party on Non Muscle Invasive Bladder Cancer aDepartment of Urology, Jeroen Bosch Hospital, Nieuwstraat 34, 5211 NL ’s-Hertogenbosch, The Netherlands bEORTC Data Center, Brussels, Belgium cDepartment of Urology, University Hospital Ghent, Ghent, Belgium dDepartment of Urology, Instituto Valenciano de Oncologia, Valencia, Spain eDepartment of Urology, Helios Agnes Karll Hospital, Bad Schwartau, Germany fDepartment of Urology, Hopital Ponchaillou, Rennes, France gDepartment of Urology, Helsinki University Hospital, Helsinki, Finland Accepted 13 May 2005 Available online 13 June 2005 Abstract Objectives: On behalf of the European Association of Urology (EAU), guidelines for the diagnosis, therapy and follow-up of patients with urothelial carcinoma in situ (CIS) have been established. Method: The recommendations in these guidelines are based on a recent comprehensive overview and meta-analysis in which two panel members have been involved (RS and AVDM). A systematic literature search was conducted using Medline, the US Physicians’ Data Query (PDQ), the Cochrane Central Register of Controlled Trials, and reference lists in trial publications and review articles. Results: Recommendations are provided for the diagnosis, conservative and radical surgical treatment, and follow- up of patients with CIS. Levels of evidence are influenced by the lack of large randomized trials in the treatment of CIS. # 2005 Elsevier B.V. All rights reserved. Keywords: Bladder cancer; Carcinoma in situ; Bacillus Calmette-Guerin; Chemotherapy; Diagnosis; Treatment; Follow up 1.