Microrecording and Image-Guided Stereotactic Biopsy of Deep-Seated Brain Tumors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

History of Psychosurgery at Sainte-Anne Hospital, Paris, France, Through Translational Interactions Between Psychiatrists and Neurosurgeons
NEUROSURGICAL FOCUS Neurosurg Focus 43 (3):E9, 2017 History of psychosurgery at Sainte-Anne Hospital, Paris, France, through translational interactions between psychiatrists and neurosurgeons *Marc Zanello, MD, MSc,1,2,6 Johan Pallud, MD, PhD,1,2,6 Nicolas Baup, MD, PhD,3 Sophie Peeters, MSc,1 Baris Turak, MD,1,6 Marie Odile Krebs, MD, PhD,3,4,6 Catherine Oppenheim, MD, PhD,2,5,6 Raphael Gaillard, MD, PhD,3,4,6 and Bertrand Devaux, MD1,6 1Neurosurgery Department, 3Department of Psychiatry, Service Hospitalo-Universitaire, and 5Neuroradiology Department, Sainte-Anne Hospital; 2IMABRAIN, INSERM U894, and 4Laboratoire de Physiopathologie des Maladies Psychiatriques, Centre de Psychiatrie et Neurosciences, UMR S894; and 6University Paris Descartes, Paris, France Sainte-Anne Hospital is the largest psychiatric hospital in Paris. Its long and fascinating history began in the 18th centu- ry. In 1952, it was at Sainte-Anne Hospital that Jean Delay and Pierre Deniker used the first neuroleptic, chlorpromazine, to cure psychiatric patients, putting an end to the expansion of psychosurgery. The Department of Neuro-psychosurgery was created in 1941. The works of successive heads of the Neurosurgery Department at Sainte-Anne Hospital summa- rized the history of psychosurgery in France. Pierre Puech defined psychosurgery as the necessary cooperation between neurosurgeons and psychiatrists to treat the conditions causing psychiatric symptoms, from brain tumors to mental health disorders. He reported the results of his series of 369 cases and underlined the necessity for proper follow-up and postoperative re-education, illustrating the relative caution of French neurosurgeons concerning psychosurgery. Marcel David and his assistants tried to follow their patients closely postoperatively; this resulted in numerous publica- tions with significant follow-up and conclusions. -

Pallidotomy and Thalamotomy
Pallidotomy and Thalamotomy Vancouver General Hospital 899 West 12th Avenue Vancouver BC V5Z 1M9 Tel: 604-875-4111 This booklet will provide information about the following Preparing for Surgey surgical procedures: Pallidotomy and Thalamotomy. Before Admission to Hospital What is a Pallidotomy? 1) Anticoagulants and other medications that thin your A pallidotomy is an operation for Parkinson’s disease blood such as Aspirin, Coumadin (Warfarin), Lovenox where a small lesion is made in the globus pallidum (an (Enoxaparin), Ticlid (Ticlopidine), Plavix (Clopidogrel) area of the brain involved with motion control). The lesion and Ginkgo must be discontinued 2 weeks before your is made by an electrode placed in the brain through a small surgery. Pradaxa (Dabigatran), Xarelto (Rivaroxaban) opening in the skull. The beneficial effects are seen on and Eliquis (Apixaban) must be discontinued 5 days the opposite side of the body, i.e. a lesion on the left side before your surgery. of your brain will help to control movement on the right 2) Since you will be having a MRI, it is important to inform side of your body. Pallidotomy will help reduce dyskinesia your neurosurgeon if you are claustrophobic, have metal (medication induced writhing), and will also improve fragments in your eye or have a pacemaker. bradykinesia (slowness). Admission to Hospital Risks Your surgeon’s office will contact you the day before your Risks include a rare chance of death (0.2%) and a low scheduled surgery to confirm the time to report to the Jim chance (7%) of weakness or blindness on the opposite side Pattison Pavilion Admitting Department. -

Reversible Signal Abnormalities in the Hippocampus and Neocortex After Prolonged Seizures
Reversible Signal Abnormalities in the Hippocampus and Neocortex after Prolonged Seizures Stephen Chan, Steven S. M. Chin, Krishnan Kartha, Douglas R. Nordli, Robert R. Goodman, Timothy A. Pedley, and Sadek K. Hilal PURPOSE: To investigate the phenomenon of reversible increased signal intensity of medial temporal lobe structures and cerebral neocortex seen on MR images of six patients with recent prolonged seizure activity. METHODS: After excluding patients with known causes of reversible signal abnormalities (such as hypertensive encephalopathy), we retrospectively reviewed the clinical findings and MR studies of six patients whose MR studies showed reversible signal abnor- malities. MR pulse sequences included T2-weighted spin-echo coronal views or conventional short-tau inversion-recovery coronal images of the temporal lobes. RESULTS: All six MR studies showed increased signal intensity within the medial temporal lobe, including the hippocampus in five studies. All follow-up MR examinations showed partial or complete resolution of the hyperin- tensity within the medial temporal lobe and the neocortex. In one patient, results of a brain biopsy revealed severe cerebral cortical gliosis. Temporal lobectomy performed 4 years later showed moderate cortical gliosis and nonspecific hippocampal cell loss and gliosis. CONCLUSION: Sig- nificant hyperintensity within the temporal lobe is demonstrable on MR images after prolonged seizure activity, suggestive of seizure-induced edema or gliosis. Damage to medial temporal lobe structures by prolonged seizure activity indicates a possible mechanism of epileptogenic disorders. Index terms: Brain, magnetic resonance; Brain, temporal lobe; Hippocampus; Seizures AJNR Am J Neuroradiol 17:1725–1731, October 1996 Prolonged seizure activity is associated with character of the acute neuronal loss in the hip- long-lasting neurologic damage and even death pocampus seen after an episode of status epi- in humans (1–3); prompt treatment is required lepticus is different from the neuronal loss and to forestall irreversible changes (4). -

Preoperative Imaging Findings in Patients Undergoing Transcranial
www.nature.com/scientificreports OPEN Preoperative imaging fndings in patients undergoing transcranial magnetic resonance imaging‑guided focused ultrasound thalamotomy Cesare Gagliardo1,4*, Roberto Cannella1,4, Giuseppe Filorizzo1, Patrizia Toia1, Giuseppe Salvaggio1, Giorgio Collura2, Antonia Pignolo1, Rosario Maugeri1, Alessandro Napoli3, Marco D’amelio1, Tommaso Vincenzo Bartolotta1, Maurizio Marrale2, Gerardo Domenico Iacopino1, Carlo Catalano3 & Massimo Midiri1 The prevalence and impact of imaging fndings detected during screening procedures in patients undergoing transcranial MR‑guided Focused Ultrasound (tcMRgFUS) thalamotomy for functional neurological disorders has not been assessed yet. This study included 90 patients who fully completed clinical and neuroradiological screenings for tcMRgFUS in a single‑center. The presence and location of preoperative imaging fndings that could impact the treatment were recorded and classifed in three diferent groups according to their relevance for the eligibility and treatment planning. Furthermore, tcMRgFUS treatments were reviewed to evaluate the number of transducer elements turned of after marking as no pass regions the depicted imaging fnding. A total of 146 preoperative imaging fndings in 79 (87.8%) patients were detected in the screening population, with a signifcant correlation with patients’ age (rho = 483, p < 0.001). With regard of the group classifcation, 119 (81.5%), 26 (17.8%) were classifed as group 1 or 2, respectively. One patient had group 3 fnding and was considered ineligible. -

ASSFN Position Statement on MR-Guided Focused Ultrasound For
ASSFN Position Statement on MR-guided Focused Ultrasound for the Management of Essential Tremor Nader Pouratian, MD, PhD Gordon Baltuch, MD, PhD W. Jeff Elias, MD Robert Gross, MD, PhD ASSFN Position Statement on MRgFUS for ET Page 2 of 8 Executive Summary Purpose of the Statement 1. To provide an evidence-based best practices summary to guide health care providers in the use of MR-guided Focused Ultrasound (MRgFUS) in the management of essential tremor (ET). 2. To establish expert consensus opinion and areas requiring additional investigation. Importance of the ASSFN Statement 1. Stereotactic and functional neurosurgeons are involved in the care of patients with advanced, medically refractory essential tremor. 2. Stereotactic and functional neurosurgeons are domain-specific experts in the specialty literature and the practical use of stereotactic procedures for the management of essential tremor and other neuropsychiatric disorders. 3. Stereotactic and functional neurosurgeons are domain-specific experts in comparative assessment of benefits, risks, and alternatives of stereotactic procedures for the management of patients with essential tremor and other neuropsychiatric diagnoses. Indications for the use of MRgFUS as a treatment option for patients with essential tremor include all of the following criteria: 1. Confirmed diagnosis of ET. 2. Failure to respond to, intolerance of, or medical contraindication to use of at least two medications for ET, one of which must be a first line medication. 3. Appendicular tremor that interferes with quality of life based on clinical history. 4. Unilateral treatment. Contraindication to use of MRgFUS: 1. Bilateral MRgFUS thalamotomy. 2. Contralateral to a previous thalamotomy. 3. Cannot undergo MRI due to medical reasons. -

Infection on Neurological Implanted Devices
ECCMID Amsterdam 09.04.2016 Challenging complex infections for ID physicians Infection on neurological implanted devices Anna Conen, MD MSc Deputy Head Physician Division of Infectious Diseases and Hospital Hygiene ESCMIDKantonsspital Aarau, eLibrary Switzerland by author Disclosures Received travel grants from Gilead, Merck Sharp Dohme, ViiV Healthcare, Bristol- Myers Squibb and Janssen. ESCMID eLibrary by author Outline • Diagnosis of implant-associated infections • Treatment concepts of implant-associated infections • Specific infections associated with the following implants: Craniotomy/bone flap Cranioplasty Deep brain stimulator Ventriculo-peritoneal shunt Neurological implants ESCMIDSpinal cord stimulator External ventricular eLibrary drainage Ventriculo-atrial shunt by author Risk of implant-associated infections Device No. inserted in the US, Infection rate, % per year Fracture fixation devices 2,000,000 5–10 Dental implants 1,000,000 5–10 Joint prostheses 600,000 1–3 Neurosurgical implants 450,000 3–15 Cardiac pacemakers 300,000 1–7 Mammary implants 130,000 1–2 Mechanical heart valves 85,000 1–3 Penile implants 15,000 1–3 Heart assist devices 700 25–50 ESCMID eLibraryDarouiche RO. Clin Infect Dis 2011; 33:1567-1572 by author Concept and diagnosis of biofilm Biofilm Sonication - Bacteria adhere to implant - Sonication of implants*: surface detachment of biofilm - Embed in a matrix - Sonication fluid plated on - In stationary growth phase culture media - Slowly replicate Standard method: 3 Sonication of tissue biopsies implant: Sensitivity ~60% Sensitivity 80-90% *Cranioplasty, shunts, screws, plates, stimulators, etc. Zimmerli W. J Infect Dis. 1982;146(4):487-97. Trampuz A. NEJM 2007;357:654–663. Portillo M. J Clin Microbiol 2015;53(5):1622-7. -
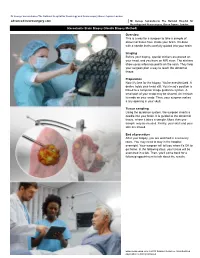
Advanced-Neurosurgery.Com Stereotactic
Dr George Samandouras The National Hospital for Neurology and Neurosurgery Queen Square London advanced-neurosurgery.com Mr George Samandouras The National Hospital for Neurology and Neurosurgery, Queen Square, London Stereotactic Brain Biopsy (Needle Biopsy Method) Overview This is a way for a surgeon to take a sample of abnormal tissue from inside your brain. It's done with a needle that's carefully guided into your brain. Imaging Before your biopsy, special stickers are placed on your head, and you have an MRI scan. The stickers show up as reference points on the scan. They help your surgeon plan a way to reach the abnormal tissue. Preparation Now it's time for the biopsy. You're anesthetized. A device holds your head still. Your head's position is linked to a computer image guidance system. A small part of your scalp may be shaved. An incision is made on your scalp. Then, your surgeon makes a tiny opening in your skull. Tissue sampling Using the guidance system, the surgeon inserts a needle into your brain. It is guided to the abnormal tissue, where it takes a sample. More than one sample may be needed. Finally, your skull and your skin are closed. End of procedure After your biopsy, you are watched in a recovery room. You may need to stay in the hospital overnight. Your surgeon will tell you when it's OK to go home. In the following days, your tissue will be examined in a lab. Then, you'll come back for a followup appointment to talk about the results. -

Surgical Management of Brain Tumors
SURGICAL MANAGEMENT OF BRAIN TUMORS JASSIN M. JOURIA, MD DR. JASSIN M. JOURIA IS A MEDICAL DOCTOR, PROFESSOR OF ACADEMIC MEDICINE, AND MEDICAL AUTHOR. HE GRADUATED FROM ROSS UNIVERSITY SCHOOL OF MEDICINE AND HAS COMPLETED HIS CLINICAL CLERKSHIP TRAINING IN VARIOUS TEACHING HOSPITALS THROUGHOUT NEW YORK, INCLUDING KING’S COUNTY HOSPITAL CENTER AND BROOKDALE MEDICAL CENTER, AMONG OTHERS. DR. JOURIA HAS PASSED ALL USMLE MEDICAL BOARD EXAMS, AND HAS SERVED AS A TEST PREP TUTOR AND INSTRUCTOR FOR KAPLAN. HE HAS DEVELOPED SEVERAL MEDICAL COURSES AND CURRICULA FOR A VARIETY OF EDUCATIONAL INSTITUTIONS. DR. JOURIA HAS ALSO SERVED ON MULTIPLE LEVELS IN THE ACADEMIC FIELD INCLUDING FACULTY MEMBER AND DEPARTMENT CHAIR. DR. JOURIA CONTINUES TO SERVES AS A SUBJECT MATTER EXPERT FOR SEVERAL CONTINUING EDUCATION ORGANIZATIONS COVERING MULTIPLE BASIC MEDICAL SCIENCES. HE HAS ALSO DEVELOPED SEVERAL CONTINUING MEDICAL EDUCATION COURSES COVERING VARIOUS TOPICS IN CLINICAL MEDICINE. RECENTLY, DR. JOURIA HAS BEEN CONTRACTED BY THE UNIVERSITY OF MIAMI/JACKSON MEMORIAL HOSPITAL’S DEPARTMENT OF SURGERY TO DEVELOP AN E- MODULE TRAINING SERIES FOR TRAUMA PATIENT MANAGEMENT. DR. JOURIA IS CURRENTLY AUTHORING AN ACADEMIC TEXTBOOK ON HUMAN ANATOMY & PHYSIOLOGY. Abstract The field of brain tumor research, diagnosis, and treatment is rapidly evolving. Over 120 types of brain tumors have been identified to date, and that number continues to increase. As the information available about brain tumors grows, so does the ability to target screening and therapies to provide patients with optimal outcomes. It is critical that health clinicians understand the surgical and treatment options in order to educate patients and to develop a care plan that has a positive outcome while respecting the patient's needs and desires. -

Transmissible Spongiform Encephalopathies (TSE) Including 7.12.18 - Revised Creutzfeldt - Jakob Disease (CJD) 2002 - Author
Section: UTMB On-line Documentation 01.37 - Policy Subject: Infection Control & Healthcare Epidemiology Policies and Procedures Topic: 01.37 - Transmissible Spongiform Encephalopathies (TSE) including 7.12.18 - Revised Creutzfeldt - Jakob Disease (CJD) 2002 - Author 01.37 - Transmissible Spongiform Encephalopathies (TSE) including Creutzfeldt - Jakob Disease (CJD) Purpose To protect healthcare workers from TSE Audience All employees of UTMB hospitals, clinics, contract workers, volunteers, and students At Risk Patients The following patients are considered to be at risk for TSE: Patients with rapidly progressive dementia, ataxia, and myoclonus. Patients who have received cadaver-derived hormones, especially growth hormones before 1970. Healthcare Epidemiology should be notified about all patients considered at high risk for TSE. Table 2 Distribution of Infectivity in the human body1 Infectivity Category Tissues, Secretions, and Excretions High Infectivity Brain Spinal Cord Eye Low Infectivity CSF Kidney Liver Lung Lymph nodes/spleen Placenta No Detectable Infectivity Adipose tissue Adrenal gland Gingival tissue Heart muscle Intestine Peripheral nerve Prostate Skeletal muscle Testis Thyroid gland Tears Nasal mucous Saliva Sweat Serous exudate Milk Semen Urine Feces Blood Page 1 of 9 Section: UTMB On-line Documentation 01.37 - Policy Subject: Infection Control & Healthcare Epidemiology Policies and Procedures Topic: 01.37 - Transmissible Spongiform Encephalopathies (TSE) including 7.12.18 - Revised Creutzfeldt - Jakob Disease (CJD) 2002 - Author 1 Assignment of different organs and tissues to categories of high and low infectivity is chiefly based upon the frequency with which infectivity has been detectable, rather than upon quantitative assays of the level of infectivity, for which data are incomplete. Experimental data include primates inoculated with tissues from human cases of CJD, but have been supplemented in some categories by data obtained from naturally occurring animal TSEs. -

Functional Neurosurgery: Movement Disorder Surgery
FunctionalFunctional Neurosurgery:Neurosurgery: MovementMovement DisorderDisorder SurgerySurgery KimKim J.J. Burchiel,Burchiel, M.D.,M.D., F.A.C.S.F.A.C.S. DepartmentDepartment ofof NeurologicalNeurological SurgerySurgery OregonOregon HealthHealth andand ScienceScience UniversityUniversity MovementMovement DisorderDisorder SurgerySurgery •• New New resultsresults ofof anan OHSUOHSU StudyStudy –– Thalamotomy Thalamotomy v. v. DBSDBS forfor TremorTremor •• Latest Latest resultsresults ofof thethe VA/NIHVA/NIH trialtrial forfor DBSDBS Parkinson’sParkinson’s DiseaseDisease •• New New datadata onon thethe physiologyphysiology ofof DBSDBS •• The The futurefuture –– DBS DBS –– Movement Movement disorderdisorder surgerysurgery MovementMovement DisorderDisorder SurgerySurgery 1950’s1950’s :: PallidotomyPallidotomy 1960’s:1960’s: PallidotomyPallidotomy replaced replaced byby ThalamotomyThalamotomy 1970’s:1970’s: TheThe LevodopaLevodopa era era 1980’s:1980’s: ThalamicThalamic stimulationstimulation forfor tremortremor 1990’s:1990’s: Pallidotomy/thalamotomyPallidotomy/thalamotomy rediscovered rediscovered 2000’s:2000’s: STNSTN andand GPiGPi stimulation stimulation 2010’s2010’s andand beyond:beyond: 99 DiffusionDiffusion catheterscatheters forfor trophictrophic factors? factors? 99 TransplantationTransplantation ofof engineeredengineered cells?cells? 99 GeneGene therapy?therapy? TreatmentTreatment ofof Parkinson’sParkinson’s DiseaseDisease •• Symptomatic Symptomatic –– Therapies Therapies toto helphelp thethe symptomssymptoms ofof PDPD •Medicine•Medicine -

Techniques for the Application of Stereotactic Head Frames Based on a 25-Year Experience
Open Access Technical Report DOI: 10.7759/cureus.543 Techniques for the Application of Stereotactic Head Frames Based on a 25-Year Experience Michael Safaee 1 , John Burke 1 , Michael W. McDermott 1 1. Department of Neurological Surgery, University of California, San Francisco Corresponding author: Michael Safaee, [email protected] Abstract The use of skull fixed stereotactic head frames remains an integral part of neurosurgical practice. Methods for the positioning, anesthesia, and fixation have been described in various publications. The authors describe the steps used currently that reflect a 25-year experience with stereotactic frame application. Photographs were obtained throughout the set-up and frame application process. The step-by-step methods were described with accompanying figures. Consent was obtained from that patient to allow for photographs throughout the frame application process. Consent was also obtained from a separate patient for videotaping the entire application process. Descriptive tags are embedded in the video to assist with the instruction of the senior author's (MWM) methods. The senior author has used the described method in over 1,000 cases. A recent analysis of the patient pain experience has been reported and is well tolerated. Supplemental devices beyond the manufacturers' standard equipment have been employed or developed: ethyl chloride spray, angled front posts, frame positioner, and torque wrenches. There have been no shunt perforations, no cranial vault penetrations, one titanium mesh cranioplasty deformation, three pin site infections (3 patients; 4,000 pin sites; 0.075%), and one thermal injury (0.025%). Stereotactic head frame application remains an important part of neurosurgical practice. -

Appendix V; Revised 2/28/06
CURRICULUM VITAE The Johns Hopkins University School of Medicine _____________________________ December, 2017 William S. Anderson, Ph.D., M.D. DEMOGRAPHIC AND PERSONAL INFORMATION Current Appointments 2013-present Associate Professor of Neurosurgery, The Johns Hopkins University School of Medicine 2011-present Attending Neurosurgeon, The Johns Hopkins Hospital Personal Data The Johns Hopkins Hospital Department of Neurosurgery, Meyer 8-181 600 N Wolfe Street Baltimore, MD 21287 Phone: +1(443)287-1609 Fax: +1(443)287-8044 Education and Training: Undergraduate 1990 B.S., (summa cum laude), Physics, Texas A&M University Doctoral Graduate 1992 M.A., Physics, Princeton University 1997 Ph.D., Physics, Princeton University 2001 M.D., The Johns Hopkins University Postdoctoral 2001-02 Intern, General Surgery, The Johns Hopkins Hospital 2002-08 Resident, Neurosurgery, The Johns Hopkins Hospital Professional Experience: 2008- 10 Instructor of Surgery, Harvard Medical School 2008-10 Associate Surgeon, The Brigham and Women’s Hospital 2011-13 Assistant Professor of Neurosurgery, The Johns Hopkins University School of Medicine 2011-pres Attending Neurosurgeon, The Johns Hopkins Hospital 2012-pres Core Faculty, Institute for Computational Medicine, The Johns Hopkins University, Whiting School of Engineering 2013-pres Associate Professor of Biomedical Engineering, The Johns Hopkins University RESEARCH ACTIVITIES Peer Reviewed Original Science Publications: 1. Anderson WS, Armitage JC, Dunn E, Heinrich JG, Lu C, McDonald KT, Weckel J, Zhu Y. Electron attachment, effective ionization coefficient, and electron drift velocity for CF4 gas mixtures. Nucl Instr Meth 1992;A323:273-279. 2. Young AR, Anderson WS, Calaprice FP, Cates GD, Jones GL, Krieger DA, Vogelaar RB. Laser oriented 36K for time reversal symmetry measurements.