Reversible Signal Abnormalities in the Hippocampus and Neocortex After Prolonged Seizures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Infection on Neurological Implanted Devices
ECCMID Amsterdam 09.04.2016 Challenging complex infections for ID physicians Infection on neurological implanted devices Anna Conen, MD MSc Deputy Head Physician Division of Infectious Diseases and Hospital Hygiene ESCMIDKantonsspital Aarau, eLibrary Switzerland by author Disclosures Received travel grants from Gilead, Merck Sharp Dohme, ViiV Healthcare, Bristol- Myers Squibb and Janssen. ESCMID eLibrary by author Outline • Diagnosis of implant-associated infections • Treatment concepts of implant-associated infections • Specific infections associated with the following implants: Craniotomy/bone flap Cranioplasty Deep brain stimulator Ventriculo-peritoneal shunt Neurological implants ESCMIDSpinal cord stimulator External ventricular eLibrary drainage Ventriculo-atrial shunt by author Risk of implant-associated infections Device No. inserted in the US, Infection rate, % per year Fracture fixation devices 2,000,000 5–10 Dental implants 1,000,000 5–10 Joint prostheses 600,000 1–3 Neurosurgical implants 450,000 3–15 Cardiac pacemakers 300,000 1–7 Mammary implants 130,000 1–2 Mechanical heart valves 85,000 1–3 Penile implants 15,000 1–3 Heart assist devices 700 25–50 ESCMID eLibraryDarouiche RO. Clin Infect Dis 2011; 33:1567-1572 by author Concept and diagnosis of biofilm Biofilm Sonication - Bacteria adhere to implant - Sonication of implants*: surface detachment of biofilm - Embed in a matrix - Sonication fluid plated on - In stationary growth phase culture media - Slowly replicate Standard method: 3 Sonication of tissue biopsies implant: Sensitivity ~60% Sensitivity 80-90% *Cranioplasty, shunts, screws, plates, stimulators, etc. Zimmerli W. J Infect Dis. 1982;146(4):487-97. Trampuz A. NEJM 2007;357:654–663. Portillo M. J Clin Microbiol 2015;53(5):1622-7. -

Microrecording and Image-Guided Stereotactic Biopsy of Deep-Seated Brain Tumors
CLINICAL ARTICLE J Neurosurg 123:978–988, 2015 Microrecording and image-guided stereotactic biopsy of deep-seated brain tumors Keiya Iijima, MD,1 Masafumi Hirato, MD, PhD,1 Takaaki Miyagishima, MD, PhD,1 Keishi Horiguchi, MD, PhD,1 Kenichi Sugawara, MD, PhD,1 Junko Hirato, MD, PhD,3 Hideaki Yokoo, MD, PhD,2 and Yuhei Yoshimoto, MD, PhD1 Departments of 1Neurosurgery and 2Human Pathology, Gunma University Graduate School of Medicine; and 3Clinical Department of Pathology, Gunma University Hospital, Maebashi, Gunma, Japan OBJECT Image-guided stereotactic brain tumor biopsy cannot easily obtain samples of small deep-seated tumor or se- lectively sample the most viable region of malignant tumor. Image-guided stereotactic biopsy in combination with depth microrecording was evaluated to solve such problems. METHODS Operative records, MRI findings, and pathological specimens were evaluated in 12 patients with small deep-seated brain tumor, in which image-guided stereotactic biopsy was performed with the aid of depth microrecording. The tumors were located in the caudate nucleus (1 patient), thalamus (7 patients), midbrain (2 patients), and cortex (2 patients). Surgery was performed with a frameless stereotactic system in 3 patients and with a frame-based stereotactic system in 9 patients. Microrecording was performed to study the electrical activities along the trajectory in the deep brain structures and the tumor. The correlations were studied between the electrophysiological, MRI, and pathological find- ings. Thirty-two patients with surface or large brain tumor were also studied, in whom image-guided stereotactic biopsy without microrecording was performed. RESULTS The diagnostic yield in the group with microrecording was 100% (low-grade glioma 4, high-grade glioma 4, diffuse large B-cell lymphoma 3, and germinoma 1), which was comparable to 93.8% in the group without microrecord- ing. -
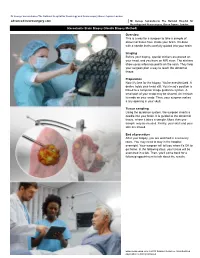
Advanced-Neurosurgery.Com Stereotactic
Dr George Samandouras The National Hospital for Neurology and Neurosurgery Queen Square London advanced-neurosurgery.com Mr George Samandouras The National Hospital for Neurology and Neurosurgery, Queen Square, London Stereotactic Brain Biopsy (Needle Biopsy Method) Overview This is a way for a surgeon to take a sample of abnormal tissue from inside your brain. It's done with a needle that's carefully guided into your brain. Imaging Before your biopsy, special stickers are placed on your head, and you have an MRI scan. The stickers show up as reference points on the scan. They help your surgeon plan a way to reach the abnormal tissue. Preparation Now it's time for the biopsy. You're anesthetized. A device holds your head still. Your head's position is linked to a computer image guidance system. A small part of your scalp may be shaved. An incision is made on your scalp. Then, your surgeon makes a tiny opening in your skull. Tissue sampling Using the guidance system, the surgeon inserts a needle into your brain. It is guided to the abnormal tissue, where it takes a sample. More than one sample may be needed. Finally, your skull and your skin are closed. End of procedure After your biopsy, you are watched in a recovery room. You may need to stay in the hospital overnight. Your surgeon will tell you when it's OK to go home. In the following days, your tissue will be examined in a lab. Then, you'll come back for a followup appointment to talk about the results. -

Surgical Management of Brain Tumors
SURGICAL MANAGEMENT OF BRAIN TUMORS JASSIN M. JOURIA, MD DR. JASSIN M. JOURIA IS A MEDICAL DOCTOR, PROFESSOR OF ACADEMIC MEDICINE, AND MEDICAL AUTHOR. HE GRADUATED FROM ROSS UNIVERSITY SCHOOL OF MEDICINE AND HAS COMPLETED HIS CLINICAL CLERKSHIP TRAINING IN VARIOUS TEACHING HOSPITALS THROUGHOUT NEW YORK, INCLUDING KING’S COUNTY HOSPITAL CENTER AND BROOKDALE MEDICAL CENTER, AMONG OTHERS. DR. JOURIA HAS PASSED ALL USMLE MEDICAL BOARD EXAMS, AND HAS SERVED AS A TEST PREP TUTOR AND INSTRUCTOR FOR KAPLAN. HE HAS DEVELOPED SEVERAL MEDICAL COURSES AND CURRICULA FOR A VARIETY OF EDUCATIONAL INSTITUTIONS. DR. JOURIA HAS ALSO SERVED ON MULTIPLE LEVELS IN THE ACADEMIC FIELD INCLUDING FACULTY MEMBER AND DEPARTMENT CHAIR. DR. JOURIA CONTINUES TO SERVES AS A SUBJECT MATTER EXPERT FOR SEVERAL CONTINUING EDUCATION ORGANIZATIONS COVERING MULTIPLE BASIC MEDICAL SCIENCES. HE HAS ALSO DEVELOPED SEVERAL CONTINUING MEDICAL EDUCATION COURSES COVERING VARIOUS TOPICS IN CLINICAL MEDICINE. RECENTLY, DR. JOURIA HAS BEEN CONTRACTED BY THE UNIVERSITY OF MIAMI/JACKSON MEMORIAL HOSPITAL’S DEPARTMENT OF SURGERY TO DEVELOP AN E- MODULE TRAINING SERIES FOR TRAUMA PATIENT MANAGEMENT. DR. JOURIA IS CURRENTLY AUTHORING AN ACADEMIC TEXTBOOK ON HUMAN ANATOMY & PHYSIOLOGY. Abstract The field of brain tumor research, diagnosis, and treatment is rapidly evolving. Over 120 types of brain tumors have been identified to date, and that number continues to increase. As the information available about brain tumors grows, so does the ability to target screening and therapies to provide patients with optimal outcomes. It is critical that health clinicians understand the surgical and treatment options in order to educate patients and to develop a care plan that has a positive outcome while respecting the patient's needs and desires. -

Transmissible Spongiform Encephalopathies (TSE) Including 7.12.18 - Revised Creutzfeldt - Jakob Disease (CJD) 2002 - Author
Section: UTMB On-line Documentation 01.37 - Policy Subject: Infection Control & Healthcare Epidemiology Policies and Procedures Topic: 01.37 - Transmissible Spongiform Encephalopathies (TSE) including 7.12.18 - Revised Creutzfeldt - Jakob Disease (CJD) 2002 - Author 01.37 - Transmissible Spongiform Encephalopathies (TSE) including Creutzfeldt - Jakob Disease (CJD) Purpose To protect healthcare workers from TSE Audience All employees of UTMB hospitals, clinics, contract workers, volunteers, and students At Risk Patients The following patients are considered to be at risk for TSE: Patients with rapidly progressive dementia, ataxia, and myoclonus. Patients who have received cadaver-derived hormones, especially growth hormones before 1970. Healthcare Epidemiology should be notified about all patients considered at high risk for TSE. Table 2 Distribution of Infectivity in the human body1 Infectivity Category Tissues, Secretions, and Excretions High Infectivity Brain Spinal Cord Eye Low Infectivity CSF Kidney Liver Lung Lymph nodes/spleen Placenta No Detectable Infectivity Adipose tissue Adrenal gland Gingival tissue Heart muscle Intestine Peripheral nerve Prostate Skeletal muscle Testis Thyroid gland Tears Nasal mucous Saliva Sweat Serous exudate Milk Semen Urine Feces Blood Page 1 of 9 Section: UTMB On-line Documentation 01.37 - Policy Subject: Infection Control & Healthcare Epidemiology Policies and Procedures Topic: 01.37 - Transmissible Spongiform Encephalopathies (TSE) including 7.12.18 - Revised Creutzfeldt - Jakob Disease (CJD) 2002 - Author 1 Assignment of different organs and tissues to categories of high and low infectivity is chiefly based upon the frequency with which infectivity has been detectable, rather than upon quantitative assays of the level of infectivity, for which data are incomplete. Experimental data include primates inoculated with tissues from human cases of CJD, but have been supplemented in some categories by data obtained from naturally occurring animal TSEs. -

Techniques for the Application of Stereotactic Head Frames Based on a 25-Year Experience
Open Access Technical Report DOI: 10.7759/cureus.543 Techniques for the Application of Stereotactic Head Frames Based on a 25-Year Experience Michael Safaee 1 , John Burke 1 , Michael W. McDermott 1 1. Department of Neurological Surgery, University of California, San Francisco Corresponding author: Michael Safaee, [email protected] Abstract The use of skull fixed stereotactic head frames remains an integral part of neurosurgical practice. Methods for the positioning, anesthesia, and fixation have been described in various publications. The authors describe the steps used currently that reflect a 25-year experience with stereotactic frame application. Photographs were obtained throughout the set-up and frame application process. The step-by-step methods were described with accompanying figures. Consent was obtained from that patient to allow for photographs throughout the frame application process. Consent was also obtained from a separate patient for videotaping the entire application process. Descriptive tags are embedded in the video to assist with the instruction of the senior author's (MWM) methods. The senior author has used the described method in over 1,000 cases. A recent analysis of the patient pain experience has been reported and is well tolerated. Supplemental devices beyond the manufacturers' standard equipment have been employed or developed: ethyl chloride spray, angled front posts, frame positioner, and torque wrenches. There have been no shunt perforations, no cranial vault penetrations, one titanium mesh cranioplasty deformation, three pin site infections (3 patients; 4,000 pin sites; 0.075%), and one thermal injury (0.025%). Stereotactic head frame application remains an important part of neurosurgical practice. -

Tariff of Fees for Insured Medical Services Regulations (O.I.C. 81-379)
0 Certified to be a true copy of an Order of his Honour the Governor of Nova Scotia in Council made the EXCUTVE coUNCIL Lieutenant 24th day of March A. D. 1981 N. S. Reuhton NOVA scoriA _3i/_’) 81-379 / The Governor in Council on the report and recommendation of the Minister of Health dated the 13th day of March, A... D., 1981, and pursuant to clause (a) of subection (1) of Section 11 of Chapter 8 of the Statutes of Nova Scotia, 1973, the Health Services and Insurance Act, and subsection (3) of Section 4 of Chapter 15 of the Statutes of Nova Scotia, 1973, the Regulations Act, is pleased to: (a) approve and authorize payments by the Health Services and Insurance Commission in respect of the tariff of fees for insured medical services attached to and forming part of the report and recommendation, and marked Schedule “A”, effective for a period of one year, comrnencing.on the 1st day of April, A. D., 1980, at a rate of 85% of the fee so calculated; and (b) order that the publication of this regulation be dispense4 with due to the length of the Schedule attached to and forming part of the report and recommendation. 1-3. H F. G. STEVEiS, Q..C., CLEKOF THE EXECUTIVE COUNCIL. 5/1 SCHEDULE “A” TABLE OF CONTENTS PREAMBLE 2 UNIT VALUE SYSTEM 21 MISCELLANEOUS FEES 22 FAMILY PRACTICE 24 ANAESTHESIA 26 DERMATOLOGY 29 INTERNAL MEDICINE 31 NEUROLOGY 33 PHYSICAL MEDICINE AND REHABILITATION 35 PABDIATRICS 37 PSYCHIATRY 39 DIAGNOSTIC AND THERAPEUTIC PROCEDURES 41 GENERAL SURGERY 51 NEUROSURGERY 75 OBSTETRICS AND GYNAECOLOGY 82 OPHTHALMOLOGY 87 OTOLARYNGOLOGY 92 ORTHOPAEDIC SURGERY 98 PLASTIC SURGERY 116 UROLOGY 124 RADIATION ONCOLOGY 133 PATHOLOGY CONSULTATIONS 134 INDEX 150 —- — — e • r. -

Report: Rs04328‐R1328 North Carolina Department of Health and Human Services Physician Fee Schedule As Of:12/11/2018
REPORT: RS04328‐R1328 NORTH CAROLINA DEPARTMENT OF HEALTH AND HUMAN SERVICES PHYSICIAN FEE SCHEDULE AS OF:12/11/2018 Physician Fee Schedule Provider Specialty 001 Effective Date: 1/1/2018 The inclusion of a rate on this table does not guarantee that a service is covered. Please refer to the Medicaid Billing Guide and the Medicaid and Health Choice Clinical Policies on the DMA Web Site. Providers should always bill their usual and customary charges. Please use the monthly NC Medicaid Bulletins for additions, changes and deletion to this schedule. Medicaid Maximum Allowable NON-FACILITY PROCEDURE CODE MODIFIER PROCEDURE DESCRIPTION FACILITY RATE RATE EFFECTIVE DATE 01967 NEURAXIAL LABOR ANALGESIA/ANESTHESIA FOR $ 209.63 $ 209.63 01996 DAILY HOSPITAL MANAGEMENT OF EPIDURAL OR $ 38.93 $ 38.93 10021 FINE NEEDLE ASPIRATION; WITHOUT IMAGING $ 52.36 $ 100.48 10022 FINE NEEDLE ASPIRATION; WITH IMAGING GUI $ 51.97 $ 103.17 10030 GUIDE CATHET FLUID DRAINAGE $ 126.07 $ 615.23 10035 PERQ DEV SOFT TISS 1ST IMAG $ 74.46 $ 437.80 10036 PERQ DEV SOFT TISS ADD IMAG $ 37.49 $ 379.35 10040 ACNE SURGERY $ 63.53 $ 72.20 10060 DRAINAGE OF ABSCESS $ 67.39 $ 77.74 10061 DRAINAGE OF ABSCESS $ 120.14 $ 133.85 10080 DRAINAGE OF PILONIDAL CYST $ 68.87 $ 114.75 10081 DRAINAGE OF PILONIDAL CYST $ 120.71 $ 181.14 10120 FOREIGN BODY REMOVAL, SKIN $ 66.08 $ 94.90 10121 FOREIGN BODY REMOVAL, SKIN $ 135.29 $ 185.09 Printed 12/11/2018 Page 1 of 329 REPORT: RS04328‐R1328 NORTH CAROLINA DEPARTMENT OF HEALTH AND HUMAN SERVICES PHYSICIAN FEE SCHEDULE AS OF:12/11/2018 10140 DRAINAGE -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

Remote Transcranial Doppler Monitoring for Carotid Interventions
Remote Transcranial Doppler Monitoring for Carotid Interventions: First Demonstration of Feasibility and Efficacy Daniel Santirso, Zsolt Garami, Alan Lumsden Cardiovascular Surgery Department. Houston Methodist DeBakey Heart and Vascular Center, Houston (Texas), USA. Introduction Transcranial Doppler (TCD) is an important tool in monitoring carotid interventions. One of the limitations is the need of a Neurosonology expert during procedures. This observational study is intended to address 2 issues: 1) can the TCD-headframe be placed by a non-expert with remote advice from the expert, 2) can the remote expert observe the monitoring and communicate with the surgeon effectively. Methods With the VisitOR1® teleproctoring technology (Karl Storz Endoscope, Tuttlingen, Germany), a Neurosonology expert in a remote place, and a non-expert physician in the operating room monitored 10 carotid procedures with TCD. We registered if the non-expert was able to place headframe and obtained a proper MCA signal. We classified interactions of the remote expert as 1) major, if the interaction influenced in technical aspects of the surgery, and 2) minor, in the rest of the cases. As safety end-point, we registered stroke or death of any cause until the patient was discharged. Besides, we asked the participants to complete an experience survey. Results The monitoring was completed in 9 of the 10 cases. Mean value of major interactions per procedure was 1,22 (range 0-2) and mean value of minor interventions was 6,78 (range 1-15). No stroke or death of any cause was registered. The participating parties evaluated the experience as positive with an overall grade of satisfaction from 90% to 100%. -

Comparative Analysis of Diagnostic Accuracy of Different Brain Biopsy Procedures
Original Article Comparative analysis of diagnostic accuracy of different brain biopsy procedures Deepali Jain, Mehar Chand Sharma, Chitra Sarkar, Deepak Gupta*, Manmohan Singh*, A. K. Mahapatra* Departments of Pathology and *Neurosurgery, All India Institute of Medical Sciences, New Delhi, India Background: Image-guided procedures such as computed Key words: Diagnostic yield, frame-based, frameless, tomography (CT) guided, neuronavigator-guided and histopathology, stereotactic biopsy, ultrasound-guided ultrasound-guided methods can assist neurosurgeons in localizing the intraparenchymal lesion of the brain. However, despite improvements in the imaging techniques, an accurate diagnosis of intrinsic lesion requires tissue sampling and histological verification. Aims: The present Recently, there has been an increasing trend in most branches study was carried out to examine the reliability of the of surgery towards a reduction in the morbidity of a procedure by diagnoses made on tumor sample obtained via different limiting surgical entry. This usually involves the application of stereotactic and ultrasound-guided brain biopsy technology such as laparoscopic cholecystectomy. With regard to procedures. Materials and Methods: A retrospective brain lesion diagnosis.com). and surgery, technological advances include analysis was conducted of all brain biopsies (frame-based the use of computer-assisted stereotaxis, intraoperative and frameless stereotactic and ultrasound-guided) ultrasound, magnetic resonance imaging, brain mapping and performed in a single tertiary care neurosciences center endoscopy. between 1995 and 2005. The overall diagnostic accuracy achieved on histopathology and correlation with type of Computer-assisted stereotactic biopsy biopsy technique was evaluated. Results: A total of 130.medknow Stereotaxis refers to a system of navigating to any point within cases were included, which consisted of 82 males and 48 the brain with the aid of imaging techniques that concurrently females. -

PNEUMOENCEPHALOGRAPHY Delivery; 20 Pregnancies in a Further 18 Patients Were in DIAGNOSIS of FITS Artificially Terminated
ApRIL 19, 1952 PULMONARY TUBERCULOSIS AND PREGNANCY mum= 847 have been studied, with particular regard to the inci- dence of pregnancy and its effect on the tuberculosis. VALUE OF There were 54 completed pregnancies in 47 patients, who were followed up for at least one year after PNEUMOENCEPHALOGRAPHY delivery; 20 pregnancies in a further 18 patients were IN DIAGNOSIS OF FITS artificially terminated. BY The patients with completed and interrupted preg- nancies were each divided into two groups-those with JOHN MARSHALL, M.D., M.R.C.P. active and those with non-active disease. In each of Major, R.A.M.C. these groups the results in patients with completed and those with interrupted pregnancies did not differ appre- AND ciably at the end of the follow-up period. C. W. Me WHflTY, D.M., M.R.C.P. Of patients with persistently patent cavities in spite of (From a Military Hospital for Head Injuries, and the treatment, seven completed their pregnancies, and of Department of Neurology, Radcliffe Infirmary, these, six later deteriorated. Of five whose pregnancies Oxford) were terminated four later deteriorated. As expected, the birth rate among these patients was The occurrence of fits in a young and otherwise healthy very low, being of the order of one-quarter of that for adult who has no abnormality on clinical examination is women of the same age groups in the general popula- fairly frequent in neurological practice among the armed tion. Forces. Once there is convincing clinical evidence that The initial diagnosis of tuberculosis less than three they are genuine epileptic attacks, the further problem months after an antecedent pregnancy was not made arises whether they are idiopathic (constitutional) or with greater frequency than could be ascribed to chance.