Part 2: Application of Estuarine Waste Load Allocation Models
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
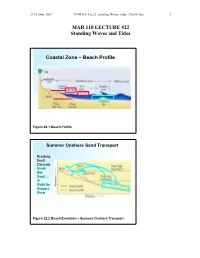
MAR 110 LECTURE #22 Standing Waves and Tides
27 October 2007 MAR110_Lec22_standing Waves_tides_27oct07.doc 1 MAR 110 LECTURE #22 Standing Waves and Tides Coastal Zone – Beach Profile Figure 22.1 Beach Profile Summer Onshore Sand Transport Breaking Swell Currents Erode Bar Sand…. & Build the Summer Berm Figure 22.2 Beach Evolution – Summer Onshore Transport 27 October 2007 MAR110_Lec22_standing Waves_tides_27oct07.doc 2 Winter Offshore Sand Transport Winter Storm Wave Currents Erode Beach Sand…. to form sandbars Figure 22.3 Beach Evolution – Winter Offshore Transport No Net Motion or Energy Propagation Figure 22.4 Wave Reflection and Standing Waves A standing wave does not travel or propagate but merely oscillates up and down with stationary nodes (with no vertical movement) and antinodes (with the maximum possible movement) that oscillates between the crest and the trough. A standing wave occurs when the wave hits a barrier such as a seawall exactly at either the wave’s crest or trough, causing the reflected wave to be a mirror image of the original. (??) 27 October 2007 MAR110_Lec22_standing Waves_tides_27oct07.doc 3 Standing Waves and a Bathtub Seiche Figure 22.5 Standing Waves Standing waves can also occur in an enclosed basin such as a bathtub. In such a case, at the center of the basin there is no vertical movement and the location of this node does not change while at either end is the maximum vertical oscillation of the water. This type of waves is also known as a seiche and occurs in harbors and in large enclosed bodies of water such as the Great Lakes. (??, ??) Standing Wave or Seiche Period l Figure 22.6 Seiche Period The wavelength of a standing wave is equal to twice the length of the basin it is in, which along with the depth (d) of the water within the basin, determines the period (T) of the wave. -

Usually Undersea Earthquakes, Landslides, Meteor Strikes. Tsunami Are Not Like Wind Driven Waves, but Are Caused by a Change in the Basin in Which the Ocean Lies
TSUNAMI Tsunami: These are caused by displacement – usually undersea earthquakes, landslides, meteor strikes. Tsunami are not like wind driven waves, but are caused by a change in the basin in which the ocean lies. Earthquakes are measured on the Richter Scale or the Mercalli Scale. The Richter Scale measures the amount of energy released whereas the Mercalli measures the amount of damage the earthquake is capable of. Here again scales are scientific artifacts produced with a specific goal in mind. Neither is right or wrong nor in conflict with the other. Tsunami do not look like breaking waves. Rather they look like an extremely high incoming tide. They appear as though someone has been adding more and more water to the ocean and the level keeps rising. Some serious Tsunamis: Santorini (Thera) An enormous volcanic eruption which produced a tsunami Somewhere around 1628 BCE Evidence from Greenland, California tree rings Climate affected – crop failure in Chine, part of Egypt impacted, (information appears on the stele of Ahmose). Some felt that this ended Minoan Civilization but archaeological evidence finds Minoan culture after the eruption. It is possible that the society was so damaged that it became perhaps too weak to defend against a very militant Mycene. There is some speculation that this eruption is the bases of Plato's Atlantis myth. Lisbon 1755 Nov, 1st at 9:40 am. (All Saints Day) Earthquake followed by a tsunami. People reported seeing the tide go out far enough to expose some ship wrecks. Churches where many had fled for protection were destroyed. Many candles which had been lit helped ignite fires all over. -

Probable Late Messinian Tsunamiites Near Monte Dei Corvi, Italy, and the Nijar Basin, Spain: Expected Architecture of Offshore Tsunami Deposits
Nat Hazards DOI 10.1007/s11069-011-9947-9 ORIGINAL PAPER Probable late Messinian tsunamiites near Monte Dei Corvi, Italy, and the Nijar Basin, Spain: expected architecture of offshore tsunami deposits Jan Smit • Cor Laffra • Karlien Meulenaars • Alessandro Montanari Received: 1 February 2010 / Accepted: 15 August 2011 Ó The Author(s) 2011. This article is published with open access at Springerlink.com Abstract Three distinct, 30- to 80-cm-thick, graded, multilayered, coarse-grained sandstone layers, intercalated in the late Messinian mudstones of the Colombacci forma- tion in Lago Mare facies of the Trave section are interpreted as tsunamiites (Ts1–Ts3). Each of these layers is sheet-like and could be followed along strike over several tens of meters. The lower two layers (Ts1–Ts2) occur in the lower part of the Colombacci for- mation and the third (Ts3) just below a conspicuous white ‘‘colombacci’’ limestone near the top of the formation. The three sandstone layers represent unique sedimentary events within the 120-m-thick San Donato-Colombacci mudstones, which contain many thin, fine- grained, possibly storm-related turbidites. Each of the three clastic layers is overall graded and strongly cross-bedded. A single layer consists of a stack of several graded sublayers that are eroded into the underlying mudstones and into each other. Absence of hummocky cross-stratification (HCS) indicates that the layers are not produced during a large, cata- strophic storm event. Current ripples such as dm-sized trough cross-beds suggest strong, prolonged, unidirectional currents, capable of carrying coarse conglomeratic sands. Climbing ripples in middle-fine sand units indicate a high suspension load settling under waning current strength. -

Wave >True One Is a Tidal Bore – Where High Tide Advances up A
Oceanography Chapter 11: Tides “Tidal” Wave >True one is a Tidal Bore – where high tide advances up a narrow river valley. ¾ Most are about 3 feet, but the biggest is 28 ft and moves at 25 mi/hr . Wavelengths of tide – are always shallow water waves Tides are periodic, short-term change in the height of the ocean surface at a particular place caused by a combination of the gravitational force of the moon and the sun and the motion of the earth. Tides are the longest of all waves and are forced waves, which are never free of the forces that create them. First studies and ties to the moon- the Greeks – Pytheas (300 BC) Math description later – Newton – 1687 Mathematical Principals of Natural Philosophy ¾ Main idea – Pull of gravity between two bodies is proportional to the masses of the bodies, but inversely proportional to the square of the distance between them. Implications: Heavy bodies attract each other more, G weakens fast with distance F = G (m1, m2) r2 But, the tides are a little different, and are expressed by T = G (m1, m2) r3 r = distance between their centers Thus, sun is 22 trillion times more massive, but 387 times farther away ¾ So, its influence is 46% that of the moon’s Newton’s Model of Tides- Equilibrium Theory ¾ Does not function ocean depth or land masses Dynamic Theory – LaPlace – accounts for these EQ Theory Earth and Moon are attracted, but inertia (the tendency of an object to move in a straight line) keeps us in balance. Insert Pretty Drawing here: ¾ Sometimes called centrifugal force Moon revolves around the earth around the center of mass, which is located about 1000 miles down Figure 11.5: Forces involved in the development of the tidal bulge – Tractive forces ¾ Points 1-2 G > Inertia (look at arrows) ¾ Points 3-4 G< Inertia Only at CE are they equal – solid Earth can’t move much, but liquid and gas can. -

Tidal Bores, Aegir, Eagre, Mascaret, Pororoca
August 9, 2011 10:41 9.75in x 6.5in b1126-ch01 Chapter 1 INTRODUCTION 1.1. PRESENTATION A tidal bore is a series of waves propagating upstream as the tidal flow turns to rising. It forms during spring tide conditions when the tidal range exceeds 4–6 m and the flood tide is confined to a narrow funnelled estuary. The estuarine zone is defined herein as a water body where the tide meets the river flow. It corresponds to the river section where the mixing of freshwater and seawater occurs. Figure 1.1 illustrates several tidal bores in France. Figure 1.1A shows a tidal bore in the Baie du Mont Saint Michel (France). The tidal bore advances in the riverbed and on the surrounding sand flats and sandbanks. Figure 1.1B presents the tidal bore of the Garonne River about 30 km upstream of Bordeaux (France). The surfers give the scale of the bore front. Figure 1.1C illustrates the formation of a tidal bore at the upstream end of a funnel-shaped bay during the early flood tide, while Fig. 1.1D shows a tidal bore propagating upstream into a small creek. The origin of the word ‘bore’ is believed to derive from the Icelandic ‘bara’ (‘billow’, ‘wave’) indicating a potentially dangerous phenomenon, i.e. a breaking tidal bore (Coates 2007). During the 19th century, the Severn tidal bore was referred to as a ‘bore’, although it was also called ‘Hygra’(Rowbotham 1983).An older name was ‘eagre’, used today for the tidal bore of the Trent River (UK). -

Tidal Bores, Aegir, Eagre, Mascaret, Pororoca: Theory and Observations." World Scientific, Singapore, 220 Pages (ISBN: 978-981-4335-41-6 / 981-4335-41-X)
CHANSON, H. (2011). "Tidal Bores, Aegir, Eagre, Mascaret, Pororoca: Theory and Observations." World Scientific, Singapore, 220 pages (ISBN: 978-981-4335-41-6 / 981-4335-41-X) TIDAL BORES, AEGIR, EAGRE, MASCARET, POROROCA: THEORY AND OBSERVATIONS by Hubert CHANSON Professor, School of Civil Engineering, School of Engineering, The University of Queensland, Brisbane QLD 4072, Australia Ph.: (61 7) 3365 3619, Fax: (61 7) 3365 4599, Email: [email protected] Url: http://www.uq.edu.au/~e2hchans/ December 2009 Tidal bores of the Garonne River (Top left), Dordogne River (Top right), Sélune River (Bottom left) and Sée River (Bottom right) in 2008 CHANSON, H. (2011). "Tidal Bores, Aegir, Eagre, Mascaret, Pororoca: Theory and Observations." World Scientific, Singapore, 220 pages (ISBN: 978-981-4335-41-6 / 981-4335-41-X) Abstract A tidal bore is a series of waves propagating upstream as the tidal flow turns to rising. It forms during spring tide conditions when the tidal range exceeds 4 to 6 m and the flood tide is confined to a narrow funnelled estuary. The existence is based upon a fragile hydrodynamic balance between the tidal amplitude, the freshwater river flow conditions and the river channel bathymetry, and it is shown that this balance may be easily disturbed by changes in boundary conditions and freshwater inflow. This book demystifies the physics of a tidal bore and it documents thoroughly the tidal bores on our Planet with reliable and accurate informations. It aims to share a passion for a beautiful, but fragile geophysical process and it is supported by over 190 illustrations and photographs. -

Tides Throughout the Day, the Level of the Sea Rises and Falls. This Rise And
Tides bore forms. A tidal bore can have a breaking crest or it can be a smooth wave. Tidal bores usually are found in places with large Throughout the day, the level of the sea rises and falls. This rise tidal ranges. When a tidal bore enters a river, its force causes water and fall in sea level is called a tide. A tide is caused by a giant in the river to reverse its flow. Waves in a tidal bore might reach 5 m wave produced by the gravitational pull of the Sun and the Moon. in height and speeds of 65 km/h. Although this wave is only 1 m or 2 m high, its wavelength is thousands of kilometers long. As the crest of this wave How does the Moon affect tides? approaches the shore, sea level seems to rise. This rise in sea level is called high tide. When the trough of this huge wave nears The Moon and the Sun exert a gravitational pull on Earth. The Sun the shore, sea level appears to drop. This drop in sea level is is much bigger than Earth, but the Moon is much closer. The Moon referred to as low tide. has a stronger pull on Earth than the Sun. Earth and the water in Earth’s oceans respond to this pull. The water bulges outward as the What is the tidal range? Moon’s gravity pulls it. This results in a high tide. The process is shown in the figure below. As Earth rotates, Earth’s surface passes through the crests and troughs of this giant wave. -

Community Vulnerability to Elevated Sea Level and Coastal Tsunami Events in Otago
Community vulnerability to elevated sea level and coastal tsunami events in Otago Otago Regional Council Private Bag 1954, 70 Stafford St, Dunedin 9054 Phone 03 474 0827 Fax 03 479 0015 Freephone 0800 474 082 www.orc.govt.nz © Copyright for this publication is held by the Otago Regional Council. This publication may be reproduced in whole or in part provided the source is fully and clearly acknowledged. ISBN: 978 0 478 37630 2 Published July 2012 Prepared by Michael Goldsmith, Manager Natural Hazards, Otago Regional Council Community vulnerability to elevated sea level and coastal tsunami events in Otago i Executive summary The Otago coastline extends 480km from Chaslands in the south to the mouth of the Waitaki River in the north. Approximately 124,000 people (64% of Otago’s population) live within five kilometres of this coastline. A number of the communities situated along the coast have a level of hazard exposure to elevated sea level (or storm surge) and tsunami events. This report assesses the vulnerability (rather than the risk) 1 of these coastal communities to these hazards. The report draws on tsunami and storm surge modelling undertaken by National Institute of Water and Atmosphere (NIWA) for the Otago Regional Council (ORC) in 2007/08, coastal topography data and local knowledge of each community. This information has been used to assess how people and the communities in which they live would be affected during credible, high magnitude tsunami and elevated sea level events. It is intended that this information will: • increase community awareness of elevated sea level and tsunami hazard • inform decision making on the development of warning systems and evacuation plans • assist with the selection of land-use planning and development controls • increase the resilience of infrastructure and utilities (‘lifelines’). -
The Tidal Bore in the Sit- Taung River a Sensitivity Analyse of the Propagation
The tidal bore in the Sit- taung River A sensitivity analyse of the propagation M.P.de Ridder Technische Universiteit Delft THETIDALBOREINTHE SITTAUNG RIVER A SENSITIVITY ANALYSE OF THE PROPAGATION by M.P.de Ridder Additional thesis Master Hydraulic Engineering and Water Resource Management at the Delft University of Technology. Student number: 4230965 Supervisor: Prof. dr. ir. Z.B. Wang Dr. ir. M.M. Rutten An electronic version of this thesis is available at http://repository.tudelft.nl/. PREFACE This thesis is part of my master Hydraulic Engineering and Water Resource Management at the TU Delft and NUS Singapore. The research is done in collaboration with the Myanmar Maritime University. I would like to thank everyone from the Myanmar Maritime University who helped me during my stay in Myanmar. Especially Khin Kyi Cin Linn for collaborating in this study. Without your help, it was not possible to do this study. I also thank my supervisors, Zheng Bing Wang and Martine Rutten, for their feedback. M.P.de Ridder Delft, January 2017 iii ABSTRACT A 2D numerical model is set-up for the tidal bore in the Sittaung River. This model is used to analyse the effect of the bottom friction, bathymetry, tidal range and river discharge on the propagation of the tidal bore. To obtain information about the tidal bore the Sittaung River is visited two times. During the second visit, depth measurements were carried out. It became clear that the tidal bore occurs only a few days after full and new moon at the village Kyaik Ka Thar. The tidal bore is also observed near Kyaik Ka Thar as an undular bore with a height of ± 0.3 m. -
Giant Waves at Lituya Bay, Alaska
Giant Waves in --·- .. -·- --·· Lituya Bay ...-----·~--- .. ·· ,. Alaska GEOLOGICAL SURVEY PROFESSIONAL PAPER 354-C n , '., Giant Waves in Lituya Bay Alaska By DON J. MILLER SHORTER. CONTRIBUTIONS TO GENERAL GEOLOGY GEOLOGICAL SURVEY PROFESSIONAL PAPER 354-C A timely account of the nature and possible causes of certain giant waves, with eyewitness reports of their destructive capacity ' UNITED STATES GOVERNMENT PRINTING OFFIC.E, WA:SHIN:GTON : 1960 ,.... n• UNITED STATES DEPARTMENT OF THE INTERIOR FRED A. SEATON, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director ··~· For sale by the Superintendent of Documents, U.S. Government Printing Office Washington 25, D.C. CONTENTS Page Abstract------------------------------------------- 51 Giant waves-Continued Introduction ____________ - _- _----------------------- 51 Waves on October 27, 1936-Continued Acknowledgments ______ -------_--------------------- 53 Effects of the waves ________________________ _ 69 Description and history of Li tuya Bay _______________ _ 53 Nature and cause of the waves ______________ _ 70 Geographic setting _____________________________ _ 53 Sudden draining of an ice-dammed body of water ______________________________ _ Geologic setting _______________________________ _ 55 71 Exploration and settlement _____________________ _ 56 Fault displacement _____________________ _ 71 Giant,vaves-------------------------------------- 57 Rockslide, avalanche, or landslide ________ _ 71 Evidence-------------------------------------- 57 Submarine sliding ______________________ -

Minimal Model for Tidal Bore Revisited OPEN ACCESS M V Berry RECEIVED 28 April 2019 H H Wills Physics Laboratory, Tyndall Avenue, Bristol BS8 1TL, United Kingdom
New J. Phys. 21 (2019) 073021 https://doi.org/10.1088/1367-2630/ab2b19 PAPER Minimal model for tidal bore revisited OPEN ACCESS M V Berry RECEIVED 28 April 2019 H H Wills Physics Laboratory, Tyndall Avenue, Bristol BS8 1TL, United Kingdom REVISED E-mail: [email protected] 1 June 2019 Keywords: wave, Hamiltonian, asymptotic, caustic, nonlinearity, Airy function ACCEPTED FOR PUBLICATION 19 June 2019 PUBLISHED 8 July 2019 Abstract This develops a recent analysis of gentle undular tidal bores (2018 New J. Phys. 20 053066) and corrects Original content from this an error. The simplest linear-wave superposition, of monochromatic waves propagating according to work may be used under the terms of the Creative the shallow-water dispersion relation, leads to a family of profiles satisfying natural tidal bore Commons Attribution 3.0 fi licence. boundary conditions, involving initial smoothed steps with different shapes. These pro les can be Any further distribution of uniformly approximated to high accuracy in terms of the integral of an Airy function with deformed this work must maintain fi attribution to the argument. For the long times corresponding to realistic bores, the pro les condense asymptotically author(s) and the title of onto the previously obtained integral-Airy function with linear argument: as the bore propagates, it the work, journal citation and DOI. forgets the shape of the initial step. The integral-Airy profile expands slowly, as the cube root of time, rather than advancing rigidly. This ‘minimal model’ leads to simple formulas for the main properties of the profile: amplitude, maximum slope, ‘wavelength’, and steepness; and an assumption about energy loss suggests how the bore weakens as it propagates. -

Real-Time Characteristics of Tidal Bore Propagation in the Qiantang River Estuary, China, Recorded by Marine Radar Ying Li 1, Do
LI, Y., PAN, D.Z., CHANSON, H., and PAN, C.H. (2019). "Real-Time Characteristics of Tidal Bore Propagation in the Qiantang River Estuary, China, Recorded by Marine Radar." Continental Shelf Research, Vol. 180, pp. 48-58 (DOI: 10.1016/j.csr.2019.04.012) (ISSN 0278-4343). Real-Time Characteristics of Tidal Bore Propagation in the Qiantang River Estuary, China, Recorded by Marine Radar Ying Li 1, Dong-Zi Pan 2, *, Hubert Chanson3 and Cun-Hong Pan 2 1 School of Surveying and municipal engineering, Zhejiang University of Water Resources and Electric Power, 583 Xuelin Street, Hangzhou 310018, China; [email protected] 2 Zhejiang Institute of Hydraulics and Estuary, 50 East Fengqi Road, Hangzhou 310020, China; [email protected] (D.P.); [email protected] (C.P.) 3 The University of Queensland, School of Civil Engineering, Brisbane, QLD 4072, Australia; [email protected] In preparation for Continental Shelf Research * Corresponding author: Dong-Zi Pan, Zhejiang Institute of Hydraulics and Estuary, 50 East Fengqi Road, Hangzhou 310020, China. Email: [email protected]; Tel.: +86-571-8643-8014. Declarations of interest: none. Abstract Quantitative real-time observations of a tidal bore in a macro-tidal estuary are difficult and dangerous, particularly in large estuaries. Mathematical and numerical models have been used to predict tidal bore advances; however, to date, there have been no validations of large-scale flow patterns. A marine radar can provide valuable real-time information on tidal bore propagation. In this paper, a template matching method using a cross-correlation algorithm was explored to estimate the evolution and celerity of a tidal bore with medium resolution marine radar images.