Patterns of Diversity of the North-Eastern Atlantic Blenniid Fish
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Campanulaceae) Based on ITS and Tranl-F Sequence Data: Implications for a Reclassification
CORE Metadata, citation and similar papers at core.ac.uk Provided by University of the Western Cape Research Repository Cupido, C. N. et al. (2013). Phylogeny of Southern African and Australasian Wahlenbergioids (Campanulaceae) based on ITS and tranL-F sequence data: implications for a reclassification. Systematic Botany, 38(2): 523 – 535 http:// doi.org/10.1600/036364413X666714 dx. Phylogeny of Southern African and Australasian Wahlenbergioids (Campanulaceae) based on ITS and trnL-F sequence data: implications for a reclassification Christopher N. Cupido , Jessica M. Prebble , and William M. M. Eddie Abstract The Campanulaceae: Wahlenbergioideae currently comprises 15 genera, one of which, Wahlenbergia, is widespread over the southern continents. Southern Africa is the region with maximum wahlenbergioid diversity with 12 genera and approximately 252 species. A second center is Australasia with 38 Wahlenbergia species. This study used a broad sample of wahlenbergioid diversity from South Africa, Australia, and New Zealand to reconstruct a phylogeny based on chloroplast trnL-F and nuclear ITS sequences. Data were analyzed separately and in combination using parsimony and Bayesian methods. The results suggest that for the wahlenbergioids to be monophyletic Wahlenbergia hederacea has to be excluded and that none of the South African, Australian or New Zealand lineages are strictly monophyletic. There are five species assemblages that are in some disagreement with current classification in the family. Wahlenbergia, Prismatocarpus and Roella are shown to be non-monophyletic and implications for a reclassification are presented. Careful consideration of morphological characters is suggested before the adjustment of generic circumscriptions can be accomplished. Recent family-wide molecular phylogenetic studies have supported the view that the Campanulaceae s.s. -

Bio 308-Course Guide
COURSE GUIDE BIO 308 BIOGEOGRAPHY Course Team Dr. Kelechi L. Njoku (Course Developer/Writer) Professor A. Adebanjo (Programme Leader)- NOUN Abiodun E. Adams (Course Coordinator)-NOUN NATIONAL OPEN UNIVERSITY OF NIGERIA BIO 308 COURSE GUIDE National Open University of Nigeria Headquarters 14/16 Ahmadu Bello Way Victoria Island Lagos Abuja Office No. 5 Dar es Salaam Street Off Aminu Kano Crescent Wuse II, Abuja e-mail: [email protected] URL: www.nou.edu.ng Published by National Open University of Nigeria Printed 2013 ISBN: 978-058-434-X All Rights Reserved Printed by: ii BIO 308 COURSE GUIDE CONTENTS PAGE Introduction ……………………………………......................... iv What you will Learn from this Course …………………............ iv Course Aims ……………………………………………............ iv Course Objectives …………………………………………....... iv Working through this Course …………………………….......... v Course Materials ………………………………………….......... v Study Units ………………………………………………......... v Textbooks and References ………………………………........... vi Assessment ……………………………………………….......... vi End of Course Examination and Grading..................................... vi Course Marking Scheme................................................................ vii Presentation Schedule.................................................................... vii Tutor-Marked Assignment ……………………………….......... vii Tutors and Tutorials....................................................................... viii iii BIO 308 COURSE GUIDE INTRODUCTION BIO 308: Biogeography is a one-semester, 2 credit- hour course in Biology. It is a 300 level, second semester undergraduate course offered to students admitted in the School of Science and Technology, School of Education who are offering Biology or related programmes. The course guide tells you briefly what the course is all about, what course materials you will be using and how you can work your way through these materials. It gives you some guidance on your Tutor- Marked Assignments. There are Self-Assessment Exercises within the body of a unit and/or at the end of each unit. -

Terrestrial Arthropods of Macaronesia
TERRESTRIAL ARTHROPODS OF MACARONESIA Biodiversity, Ecology and Evolution Title Terrestrial Artrhropods of Macaronesia - Biodiversity, Ecology and Evolution 1st edition, 2010 Editors Artur R. M. Serrano, Paulo A. V. Borges, Mário Boieiro and Pedro Oromí Sociedade Portuguesa de Entomologia Finantial support provided by Fundação para a Ciência e a Tecnologia, Portugal Project PDCT/BIA-BDE/59202/2004 Cover design by Security Print Cover photographs (and author): Desertas Islands (Photo by SPNM), Alloxantha fulva (Photo by P. Oromí) Misumena spinifera (Photo by P. Oromí), Guanchia uxoris (Photo by P. Oromí), Acrostira euphorbiae (Photo by P. Oromí), Dolichoiulus xylomistax (Photo by P. Oromí), Longitarsus isoplexidis (Photo by A. Serrano), Backcover photographs (and author): Selvagem Grande - Selvagens (Photo by SPNM), Turinyphia cavernicola (Photo by P. Borges), Herpisticus eremita (Photo by P. Oromí), Pseudoyersinia pilipes (Photo by P. Oromí), Hogna schmitzi (Photo by P. Oromí), Ischnura hastata (Photo by A. Cordero Ribera), Domene vulcanica (Photo by P. Oromí) Printed by Security Print - Sociedade de Indústria Gráfica, Lda. ISBN: 978-972-97241-2-1 Depósito Legal: XXX $POUFOUT __________________________________________________________________________________________ Preface by Antonio Machado INTRODUCTION Chapter 1 - The islands of Macaronesia, 1 J.M. Fernández-Palacios SECTION: BIODIVERSITY and CONSERVATION Chapter 2 - The provisional status of terrestrial arthropod inventories in the Macaronesian islands, 33 Jorge M. Lobo & Paulo A.V. Borges Chapter 3 - The Macaronesian province: patterns of species richness and endemism of arthropods, 49 Kostas A. Triantis, Paulo A.V. Borges, Joaquín Hortal & Robert J. Whittaker Chapter 4 - Patterns of Alpha and Beta Diversity of Epigean Arthropods at Contrasting Land-Uses of an Oceanic Island (Terceira, Azores), 73 Pedro Cardoso, Clara Gaspar, Francisco Dinis & Paulo A.V. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Euphorbiaceae)
Polish Botanical Journal 60(2): 147–161, 2015 DOI: 10.1515/pbj-2015-0024 PHYTOGEOGRAPHICAL ANALYSIS OF EUPHORBIA SUBGENUS ESULA (EUPHORBIACEAE) Dmitry V. Geltman Abstract. Euphorbia subg. Esula is one of four major clades within the genus. A geographical analysis of the 466 species in the subgenus is reported here. Every species was assigned to one of 29 geographical elements clustered in ten groups of ele- ments. This geographical analysis showed that the Tethyan group (comprising nine geographical elements) clearly dominates the subgenus and contains 260 species (55.79% of the total number of species). The most numerous geographical elements are Irano-Turanian (105 species) and Mediterranean (85). Other significant groups of elements are Boreal (91 species, 19.54%), East Asian (40 species, 8.58%), Madrean (26 species, 5.58%), Paleotropical (23 species, 4.94%) and South African (16 species, 3.43%). The area of the Tethyan floristic subkingdom is the center of the modern diversity of E. subg. Esula. It is likely that such diversity is the result of intensive speciation that took place during the Eocene–Miocene. Key words: Euphorbia subg. Esula, geographical elements, Irano-Turanian floristic region, Mediterranean floristic region, phytogeographical analysis, Tethyan floristic subkingdom Dmitry V. Geltman, Komarov Botanical Institute of the Russian Academy of Sciences, Prof. Popov Street, 2, St. Petersburg, 197376, Russia; e-mail: [email protected] Introduction genus euphorbia and its taxonomy cantly differ from traditional ones. For subgenus Esula (Riina et al. 2013), 21 sections were ac- The giant genus Euphorbia L. (Euphorbiaceae) re- cepted on the basis of analyses of the combined cently became a subject of detailed phylogenetic and ITS + ndhF dataset (Fig. -

GPS AZORES Project
GPS AZORES Project Title: Geopolitical framework of the Macaronesia region Project Coordinator: Helena Calado (Universidade dos Azores) Work Package Coordinator: Juan Luis Suarez de Vivero (Universidad de Sevilla) Authors: Elisabetta Menini, Firdaous Halim, Daniela Gabriel, Juan Luis Suarez de Vivero, Helena Calado, Fabiana Moniz, Mario Caña Varona. Submission date: 31 August 2018 Acknowledgements: Christine Ladiero for helping with statistics Citation: Menini E., Halim F., Gabriel, D., Suarez de Vivero, JL., Calado, H., Moniz, F., Caña Varona, M. 2018. Geopolitical framework of the Macaronesia region. GPS Azores project: Ponta Delgada. Page 1 This project was financed in 85% by FEDER and in 15% with regional funds through the Programa Operacional Açores 2020 (Operational Program Azores 2020), in scope of the project « GPSAZORES -ACORES-01-0145-FEDER-00002». Page 2 Contents ACRONYMS 4 1. Maritime scenario characterisation: Main political, jurisdictional and socio-economic features 5 1.1 Political geography of the region 5 1.1.1 The regional context: Macaronesia 5 1.1.2 Countries and territories 12 1.2 Maritime space 16 1.2.1 Macaronesia in the context of the United Nations Convention of the Law of the Sea 16 1.2.2 Maritime jurisdictions 19 1.2.3 Maritime borders 21 1.3 The socio-economic context: exploitation and uses of the maritime space 26 1.3.1 Demography 26 1.3.2 Economic development 27 1.3.3 Tourism 29 1.3.1 Fisheries and aquaculture 31 1.3.2 Regional maritime geo-economics 33 1.3.3 Other relevant maritime economic activities 33 -
Supporting Information
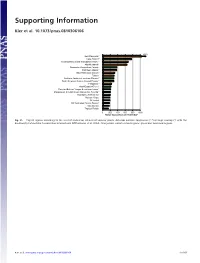
Supporting Information Kier et al. 10.1073/pnas.0810306106 1350 New Caledonia** Cape Region** Polynesia-Micronesia and Eastern Pacific** Atlantic Islands* Queensland tropical rain forests Caribbean Islands** East Melanesian Islands** Taiwan Northern Andes incl. northern Páramo** South American Atlantic Coastal Forests** Philippines** West-Ecuador/Choco* Peruvian/Bolivian Yungas & montane forests* Madagascar & Indian Ocean Islands incl. Socotra** Mountains of SW China** Western Ghats* Sri Lanka* SW Australian Floristic Region** New Guinea Tropical Florida 0 200 400 600 800 1000 Range equivalents per 10,000 km² Fig. S1. Top 20 regions according to the level of endemism richness of vascular plants. Asterisks indicate congruence (**) or large overlap (*) with the biodiversity hotspots by Conservation International (Mittermeier, et al. 2004). Orange bars indicate island regions, green bars mainland regions. Kier et al. www.pnas.org/cgi/content/short/0810306106 1of15 Fig. S2. Endemism richness (range equivalents per 10,000 km2) of terrestrial vertebrates at the ecoregion level of mainland (green) and island regions (orange). Boxes mark second and third quartiles, whiskers the nonoutlier range of the data. (A) Terrestrial vertebrates, (B) amphibians, (C) birds, (D) reptiles, and (E) mammals. Kier et al. www.pnas.org/cgi/content/short/0810306106 2of15 Spearman rank correlation Vascular 0.75 0.82 0.73 0.78 0.83 plants 90 Amphibians 0.76 0.82 0.82 0.87 0 90 Reptiles 0.85 0.85 0.94 ER rank ER rank 0 90 Birds 0.89 0.96 0 90 Mammals 0.94 0 90 Tetrapods ER rank ER rank ER rank 0 090090090090090 ER rank ER rank ER rank ER rank ER rank Fig. -

Laurus L., Lauraceae
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by idUS. Depósito de Investigación Universidad de Sevilla SPECIAL Late Neogene history of the laurel tree ISSUE (Laurus L., Lauraceae) based on phylogeographical analyses of Mediterranean and Macaronesian populations Francisco Rodrı´guez-Sa´nchez1*, Beatriz Guzma´n2, Alfredo Valido3, Pablo Vargas2 and Juan Arroyo1 1Departamento de Biologı´a Vegetal y Ecologı´a, ABSTRACT Universidad de Sevilla, Sevilla, 2Real Jardı´n Aim The post-glacial range dynamics of many European plant species have been Bota´nico, CSIC, Madrid and 3A´rea de Biodiversidad y Conservacio´n, Universidad Rey widely investigated, but information rapidly diminishes as one moves further Juan Carlos, Madrid and Integrative Ecology back in time. Here we infer the historical range shifts of Laurus, a paradigmatic Group, Estacio´n Biolo´gica de Don˜ana, CSIC, tree of the Tethyan flora that has covered southern Eurasia since the Sevilla, Spain Oligo-Miocene, by means of phylogenetic and phylogeographical analyses. Location Mediterranean Basin, Black Sea and Macaronesian archipelagos (Azores, Madeira, Canary Islands). Methods We analysed plastid DNA (cpDNA) sequence (trnK–matK, trnD–trnT) variation in 57 populations of Laurus and three Lauraceae genera. Phylogenetic methods (maximum parsimony and Bayesian inference) and statistical parsimony networks were used to reconstruct relationships among haplotypes. These results were contrasted with the fossil record and bioclimatic niche-based model predictions of past distributions to infer the migration routes and location of refugia. Results The phylogenetic tree revealed monophyly for Laurus. Overall sequence variability was low within Laurus, but six different haplotypes were distinguished and a single network retrieved, portraying three lineages primarily related to geography. -

Macaronesia Region
MACARONESIA Facts & figures Geography Total area: 1,910,764 km 2 Land area: 10,571 km 2 (1%) (1) Protected land area: 4,213 km 2 (40%) (2) Total marine area: 1,900,193 km 2 (99%) (3) Exclusive economic 1,824,702 km 2 (4) zone (EEZ): (96%) Marine protected area 113,995 km2 (5) (MPA and/or MMA): (6%) Overseas Regions Azores (PT), Madeira (Governance/Dependency): (PT), Canary Islands (ES) Socio-economic facts Total population (2014): 2,609,854 (6) Population density (2014): 247 inhab/km 2 Current situation & main challenges Average annual GDP 48,569 million € (7) (2014, prel. data): Socio-economic context Main income sources: Tourism, agriculture and (7 ) fisheries The biogeographic region of Macaronesia (from the Greek words for blessed or fortunate islands) is made up of Europe's volcanic islands in the Biodiversity Northeastern Atlantic Ocean: the 3 archipelagos of Azores (PT), Madeira (PT) and the Canaries (ES). Threatened ecosystems: Except for the Canaries, all the other islands were uninhabited before the Evergreen humid forest (Laurisilva) (8) European colonizers arrived in the 15th century. Today, the region has 2.6 • Azores: 2% of the original cover million inhabitants and a density of nearly 250 persons/km2, on average. With • Madeira: 20% of the original cover a population of about 2 million people, the Canary Islands are the most • Canaries: 18% of its potential distribution populated European overseas entity. But it’s Madeira that has the highest (9) population density of Macaronesia - 323 inhabitants/km2 - while the Azores Endemic species: 5,901 stands with the lowest population density of 106 persons/km2. -

Community Structure and Habitat Preferences of Intertidal Fishes of the Eastern Canary Islands: Fuerteventura, Gran Canaria
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1996 Community Structure and Habitat Preferences of Intertidal Fishes of the Eastern Canary Islands: Fuerteventura, Gran Canaria, and Lanzarote, With a Behavioral Description of Mauligobius Maderensis (Osteichthyes: Gobiidae). Richard Patrick Cody Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Cody, Richard Patrick, "Community Structure and Habitat Preferences of Intertidal Fishes of the Eastern Canary Islands: Fuerteventura, Gran Canaria, and Lanzarote, With a Behavioral Description of Mauligobius Maderensis (Osteichthyes: Gobiidae)." (1996). LSU Historical Dissertations and Theses. 6180. https://digitalcommons.lsu.edu/gradschool_disstheses/6180 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type o f computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. -

Madeira - Native UNESCO Natural World Heritages Sites and Laurel Forest the Region of Macaronesia Comprises 12 UNESCO Biosphere Reserves
EU OVERSEAS REGIONS OF GLOBAL With the kind support of: 7IMPORTANCE Macaronesia Did you Macaronesia know? The Macaronesia region is home to 3 Madeira - Native UNESCO Natural World Heritages sites and laurel forest The region of Macaronesia comprises 12 UNESCO Biosphere Reserves. (Laurisilva) at three volcanic archipelagos in the Madeira Nature There are more than 5,600 endemic species Atlantic Ocean: the Portuguese Park among the 23,000 existing marine and autonomous regions of the Azores (9 terrestrial species in Macaronesia. © Antonio Domingos Abreu islands) and Madeira (2 islands), and the Spanish autonomous community Laurisilva or laurel forest is a temperate of the Canary Islands (7 main islands). rainforest, which existed throughout Europe, but is now restricted to humid mountainous Compared to other European areas of Macaronesia. Overseas regions Macaronesia has Macaronesia is the only European Overseas a relatively high population density region, which benefits from Natura 2000, a with the Canary Islands being European network of protected areas. the most populated. The region’s economy is strongly specialized in The Azores, located at the triple junction of three tectonic plates (North American, the services sector, where tourism Eurasian and African), are some of the has a prominent role, particularly in world’s tallest mountains, measured from Madeira and the Canary Islands. In their deep base in the ocean to the peak. the Azores, agriculture and fisheries remain relevant income sources. 29 of the world’s 81 whale species are found Canary Islands around the Canary Islands – Angel shark, However, economic development critically also puts pressure on biodiversity endangered and ecosystems: Despite an © Carlos Suarez extensive network of local protected areas and Natura 2000 Territories sites, Macaronesia’s biodiversity is threatened by habitat destruction and invasive alien species. -

Conservation of the Macaronesian Endemic Species: Patterns Among Archipelagos and Taxonomic Groups Based on IUCN Lists
UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL Conservation of the Macaronesian endemic species: patterns among archipelagos and taxonomic groups based on IUCN lists Joana Mendes Casimiro Mestrado em Biologia da Conservação Versão Provisória Dissertação orientada por: Professora Doutora Maria Manuel Romeiras Professora Doutora Maria Filomena de Magalhães 2017 Agradecimentos A realização desta dissertação de mestrado contou com apoios indispensáveis aos quais estarei eternamente grata e sem os quais nada teria sido possível. Às minhas orientadoras, Professora Doutora Maria Manuel Romeiras e Professora Doutora Maria Filomena Magalhães, pelo incansável apoio, disponibilidade, opiniões e críticas, pelo conhecimento e segurança que transmitiram e, principalmente, pela verdadeira orientação ao longo de todo o trabalho. Ao Professor Doutor Paulo Borges pela disponibilidade e pela contribuição de dados dos artrópodes dos Açores que se mostraram essenciais para enriquecer este trabalho. À Doutora Sílvia Catarino pela disponibilização de informação sobre a biodiversidade de Cabo Verde. Finalmente, aos meus pais, Elizabete e Carlos, e à minha irmã, Rita, por não me deixarem desistir mesmo quando tudo parecia desmoronar-se e por acreditarem nas minhas capacidades, por vezes mais do que eu própria acredito, o que me levou a querer alcançar mais uma etapa. II Abstract Earth is facing one irreversible and concerning global environmental change: the loss of biodiversity. Several studies have been done in recent years in order to protect biodiversity but it is still necessary to improve global understanding on this theme. This is a very concerning situation, especially when it comes to oceanic islands, which account for only about 5% of the Earth’s surface but contain 20% of the world's biodiversity and are centers of endemism.