COMMENTARY the Diversity of Hydrostatic Skeletons
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Sirenian Feeding Apparatus: Functional Morphology of Feeding Involving Perioral Bristles and Associated Structures
THE SIRENIAN FEEDING APPARATUS: FUNCTIONAL MORPHOLOGY OF FEEDING INVOLVING PERIORAL BRISTLES AND ASSOCIATED STRUCTURES By CHRISTOPHER DOUGLAS MARSHALL A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNrVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REOUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 1997 DEDICATION to us simply as I dedicate this work to the memory of J. Rooker (known "Rooker") and to sirenian conservation. Rooker was a subject involved in the study during the 1993 sampling year at Lowry Park Zoological Gardens. Rooker died during the red tide event in May of 1996; approximately 140 other manatees also died. During his rehabilitation at Lowry Park Zoo, Rooker provided much information regarding the mechanism of manatee feeding and use of the perioral bristles. The "mortality incident" involving the red tide event in southwest Florida during the summer of 1996 should serve as a reminder that the Florida manatee population and the status of all sirenians is precarious. Although some estimates suggest that the Florida manatee population may be stable, annual mortality numbers as well as habitat degradation continue to increase. Sirenian conservation and research efforts must continue. ii ACKNOWLEDGMENTS Research involving Florida manatees required that I work with several different government agencies and private parks. The staff of the Sirenia Project, U.S. Geological Service, Biological Resources Division - Florida Caribbean Science Center has been most helpful in conducting the behavioral aspect of this research and allowed this work to occur under their permit (U.S. Fish and Wildlife Permit number PRT-791721). Numerous conversations regarding manatee biology with Dr. -

Characterization of Arm Autotomy in the Octopus, Abdopus Aculeatus (D’Orbigny, 1834)
Characterization of Arm Autotomy in the Octopus, Abdopus aculeatus (d’Orbigny, 1834) By Jean Sagman Alupay A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Integrative Biology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Roy L. Caldwell, Chair Professor David Lindberg Professor Damian Elias Fall 2013 ABSTRACT Characterization of Arm Autotomy in the Octopus, Abdopus aculeatus (d’Orbigny, 1834) By Jean Sagman Alupay Doctor of Philosophy in Integrative Biology University of California, Berkeley Professor Roy L. Caldwell, Chair Autotomy is the shedding of a body part as a means of secondary defense against a predator that has already made contact with the organism. This defense mechanism has been widely studied in a few model taxa, specifically lizards, a few groups of arthropods, and some echinoderms. All of these model organisms have a hard endo- or exo-skeleton surrounding the autotomized body part. There are several animals that are capable of autotomizing a limb but do not exhibit the same biological trends that these model organisms have in common. As a result, the mechanisms that underlie autotomy in the hard-bodied animals may not apply for soft bodied organisms. A behavioral ecology approach was used to study arm autotomy in the octopus, Abdopus aculeatus. Investigations concentrated on understanding the mechanistic underpinnings and adaptive value of autotomy in this soft-bodied animal. A. aculeatus was observed in the field on Mactan Island, Philippines in the dry and wet seasons, and compared with populations previously studied in Indonesia. -
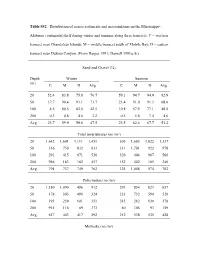
Table S32. Distribution of Coarse Sediments and Macroinfauna on the Mississippi
Table S32. Distribution of coarse sediments and macroinfauna on the Mississippi- Alabama continental shelf during winter and summer along three transects. C = western transect near Chandeleur Islands; M = middle transect south of Mobile Bay; D = eastern transect near DeSoto Canyon. (From Harper 1991; Darnell 1991a, b.) Sand and Gravel (%) Depth Winter Summer (m) C M D Avg. C M D Avg. 20 52.4 83.8 75.8 70.7 59.1 94.7 94.9 82.9 50 37.7 90.4 93.1 73.7 23.4 91.0 91.3 68.6 100 4.5 60.5 62.6 42.5 10.8 57.9 77.1 48.6 200 0.3 4.8 4.6 3.2 0.5 5.8 7.4 4.6 Avg. 23.7 59.9 59.0 47.5 23.5 62.4 67.7 51.2 Total invertebrates (no./m²) 20 1,642 1,601 1,111 1,451 505 1,683 1,822 1,337 50 316 750 832 633 311 1,701 922 978 100 291 415 871 526 326 404 967 566 200 946 183 182 457 152 402 185 246 Avg. 794 737 749 762 324 1,048 974 782 Polychaetes (no./m²) 20 1,180 1,090 486 912 293 854 823 657 50 178 305 490 324 233 732 594 520 100 193 259 601 351 243 262 630 378 200 915 116 89 373 80 305 93 159 Avg. 617 443 417 492 212 538 535 428 Mollusks (no./m²) 20 123 127 174 141 89 527 407 341 50 46 214 115 125 11 653 73 246 100 32 80 23 45 20 63 38 40 200 14 7 10 10 26 27 9 21 Avg. -

This Article Was Originally Published in the Encyclopedia of Neuroscience
This article was originally published in the Encyclopedia of Neuroscience published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non- commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who you know, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial Yekutieli Y, Flash T and Hochner B (2009) Biomechanics: Hydroskeletal. In: Squire LR (ed.) Encyclopedia of Neuroscience, volume 2, pp. 189-200. Oxford: Academic Press. Author's personal copy Biomechanics: Hydroskeletal 189 Biomechanics: Hydroskeletal Y Yekutieli and T Flash, Weizmann Institute of elephant trunk. A hydrostatic skeleton may be used Science, Rehovot, Israel in combination with a rigid skeletal support (e.g., B Hochner , Hebrew University, Jerusalem, Israel the vertebrate tongue). In this article we describe the ã 2009 Elsevier Ltd. All rights reserved. biomechanics and movement control of hydrostatic skeletons, giving examples of the diversity and unique adaptations. Type of Skeletal Systems Basic Structure of Hydrostatic Skeletons The combination of muscles (force production mech- Fluid-Filled Cavity Skeletons anisms) and skeletons enable animals to move in a variety of ways. There are basically three types Animals with FFC hydrostatic skeletons usually com- of skeletons: endoskeletons, the rigid internal skele- prise both circular and longitudinal muscle fibers, ton of vertebrates; exoskeletons, the rigid external and their shapes are typically cylindrical. -

Niche Modeling Remarks of Luidia Senegalensis (Lamarck, 1816) (Asteroidea, Luidiidae) After 30 Years of Its First Capture in the Northeastern Brazilian Coast
Latin American Journal of Aquatic Research, 48Niche(3): 497 modeling-505, 2020 remarks of Luidia senegalensis 497 DOI: 10.3856/vol48-issue3-fulltext-2130 Short communication Niche modeling remarks of Luidia senegalensis (Lamarck, 1816) (Asteroidea, Luidiidae) after 30 years of its first capture in the northeastern Brazilian coast Carolina T. Puppin-Gonçalves1, Matheus Arthur L. Rocha1, Carlos E.R.D. Alencar1,2 1 1,3 1 Sávio A.S.N. Moraes , Paulo V.N. Araújo & Fúlvio A.M. Freire 1Laboratório de Ecologia e Evolução de Crustáceos (LABEEC), Departamento de Biologia Ecologia e Zoologia, Centro de Biociências, Universidade Federal do Rio Grande do Norte (UFRN) Campus Universitário Lagoa Nova, Natal, Brasil 2Departamento de Ciências Biológicas, Universidade Regional do Cariri (URCA) Campus Pimenta Crato, Brasil 3Instituto Federal de Educação, Ciência e Tecnologia do Rio Grande do Norte (IFRN) Campus Macau, Macau, Brasil Corresponding author: Carolina T. Puppin-Gonçalves ([email protected]) ABSTRACT. After more than 30 years of species' first capture on the Brazilian northeast coast, we report the second occurrence of the starfish Luidia senegalensis with niche modeling remarks on its distribution. Bottom trawl net collected specimens with artisanal fishery boat in Rio Grande do Norte State, northeast Brazil. It was noted the existence of a large number of regions, with high suitability for the occurrence of this species, in South America taking into account the ecological niche modeling, when compared to North and Central American continents. Benthic salinity range, calcite, and benthic minimum temperature were the most relevant for modeling. The northeastern, eastern, and southeastern Brazil ecoregions showed the most considerable amount of areas with high suitability for L. -

FEP Volume II Calico Scallop
To date there has been no attempt at a comprehensive stock assessment for wahoo. Therefore, the status of the stocks is unknown at this time. Proxy MSY estimates were provided by the NMFS SEFSC and were used to specify the status determination criteria in the Dolphin Wahoo FMP. 4.1.9 Calico Scallops Description and Distribution Calico scallops, Argopecten gibbus (Linnaeus 1758), are part of the bivalve mollusc family Pectinidae that contains all commercial species of scallops (Waller 1991). They are unified by series of minute denticles formed in the notch of the right valve, most visible in early juvenile stages. Waller (2006) indicates there are four major groupings or subfamilies, three of which are monophyletic (Camptonectinae, Palliolinae and Pectininae) and one of which is paraphyletic (Chlamydinae). At least six species in the subfamily Pectininae are commercially exploited: Aequipecten operularis (queen scallop), Argopecten irradians (bay scallops) and A. gibbus in the North Atlantic, Aequipecten tehuelchus (Tehuleche scallop) in the South Atlantic, and Argopecten purpuratus (Chilean scallop) and A. ventricosus (Catarina scallop) in the eastern Pacific. Identification of calico scallops can be made from shell color and morphology. The upper (left) valve has red or maroon calico markings over a white or yellow base; the lower (right valve) is more lightly pigmented. The calico markings on the shell distinguish this scallop from the solid gray or brown upper valve of the bay scallop, which resembles the calico scallop in size. Calico scallop shell morphology varies with locality (Krause et al. 1994), but generally the species reaches 40 to 60 mm (1.6-2.4 in) in shell height (a straight line measurement of the greatest distance between the umbo and the ventral margin), with a maximum size reported to be about 80 mm (3.2 in) in shell diameter (a straight line measurement of the greatest distance between the anterior and posterior margin) (Roe et al. -

4-Araujo 64.Indd
VIE ET MILIEU - LIFE AND ENVIRONMENT, 2014, 64: 35-46 A trophic ANalYsis of target species of macrobeNthos IN A SUBTROPICAL coastal COMMUNITY: A taXA relatioNSHIP ESSAY M. E. DE ARAÚJO 1, M. J. LUNARDON-BRANCO 2, J. R. VERANI 3, J. O. BRANCO 2, J. P. BARREIROS 4* & M. L. CHRISTOFFERSEN 5 1 Universidade Federal de Pernambuco, CTG, Departamento de Oceanografia, CEP 50.740-550, Recife, Pernambuco, Brazil 2 Centro de Ciências Tecnológicas da Terra e do Mar, Universidade Vale do Itajaí, CP 360, CEP 88.302-202, Itajaí, Santa Catarina, Brazil 3 Universidade Federal de São Carlos. Cx. Postal 676, CEP 13565-905 São Carlos, SP, Brazil 4 Azorean Biodiversity Group (CITA-A) and Platform for Enhancing Ecological Research & Sustainability (PEERS), Universidade dos Açores, Dep. Ciências Agrárias, 9700-042 Angra do Heroísmo, Portugal 5 Universidade Federal da Paraíba, Departamento de Sistemática e Ecologia, CEP 58.059-900, João Pessoa, Paraíba, Brazil * Corresponding author: [email protected] CLADOGRAM ABSTRACT. – Studies on the feeding habits of aquatic organisms are a requirement for the COMMON FOOD ITEMS TROPHIC RELATIONS management and sustainable use of marine ecosystems. The aim of the present research was to analyze the habits and trophic similarities of decapods, starfish and fish in order to propose trophic relationships between taxa, using Hennigian methods of phylogenetic systematics. This new grouping hypothesis, based on shared and exclusive food items and food types, corresponds to the broad taxonomic groups used in the analysis. Our results indicate that algae, Mollusca, Polychaeta, Crustacea, Echinodermata and Actinopterygii are the most exploited common resources among the species studied. -

Non-Genetic Inheritance and Changing Environments
NON-GENETIC INHERITANCE Mini-review • DOI: 10.2478/ngi-2013-0005 • NGI • 2012 • 38-50 Non-genetic inheritance and changing environments Abstract Santiago Salinas1*, Marc Mangel1,3, Climate change continues to impact species worldwide. Understanding Simon C. Brown2, Stephan B. Munch4 and predicting how populations will respond is of clear importance. Here, we review a mechanism by which populations may respond rapidly to these changes: Trans-Generational Plasticity (TGP). TGP exists when the 1Center for Stock Assessment Research, 3Dept. of Biology, University of Bergen, environment experienced by the parents affects the shape of the reaction University of California Santa Cruz, Bergen, 5020, Norway norm in their offspring; that is, the parental and offspring environments Santa Cruz, CA 95060, USA interact to determine the offspring phenotype. We survey 80 empirical 4 2Dept. of Ecology and Evolutionary Biology, Fisheries Ecology Division, studies from 63 species (32 orders, 9 phyla) that demonstrate TGP. Overall, University of California Santa Cruz, Southwest Fisheries Science Center, TGP is taxonomically widespread and present in response to environmental Santa Cruz, CA, 95060, USA Santa Cruz, CA, 95060, USA drivers likely to be impacted by climate change. Although many examples now exist, we also identify areas of research that could greatly improve our understanding of TGP. We conclude that TGP is sufficiently established both theoretically and empirically to merit study as a potential coping tactic against rapid environmental changes. -

Mass Mortality of the Sea Stars Luidia Clathrata and Luidia Alternata Alternata on the Alabama Coast, December 2013 James B
Gulf of Mexico Science Volume 31 Article 7 Number 1 Number 1/2 (Combined Issue) 2013 Mass Mortality of the Sea Stars Luidia clathrata and Luidia alternata alternata on the Alabama Coast, December 2013 James B. McClintock University of Alabama at Birmingham Luke M. McClintock University of Alabama at Birmingham John M. Lawrence University of South Florida DOI: 10.18785/goms.3101.07 Follow this and additional works at: https://aquila.usm.edu/goms Recommended Citation McClintock, J. B., L. M. McClintock and J. M. Lawrence. 2013. Mass Mortality of the Sea Stars Luidia clathrata and Luidia alternata alternata on the Alabama Coast, December 2013. Gulf of Mexico Science 31 (1). Retrieved from https://aquila.usm.edu/goms/vol31/iss1/7 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf of Mexico Science by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. McClintock et al.: Mass Mortality of the Sea Stars Luidia clathrata and Luidia alter SHORT PAPERS AND NOTES Gulf of Mexico Science, 2013(1–2), pp. 74–78 in the web-based London Daily Mail read ‘‘Thou- E 2013 by the Marine Environmental Sciences Consortium of Alabama sands of dead starfish wash up on Lincolnshire Beach following stormy weather.’’ The ensuing MASS MORTALITY OF THE SEA STARS LUI- news article and photograph reported a mass DIA CLATHRATA AND LUIDIA ALTERNATA mortality of what was identified by a local expert ALTERNATA ON THE ALABAMA COAST, to be Asterias rubens presumably caused by strong DECEMBER 2013.—Mass mortalities among storm-generated currents (Reynolds, 2014). -

Transportation and Dispersal of Biogenic Material in the Nearshore Marine Environment
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1974 Transportation and Dispersal of Biogenic Material in the Nearshore Marine Environment. Macomb Trezevant Jervey Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Jervey, Macomb Trezevant, "Transportation and Dispersal of Biogenic Material in the Nearshore Marine Environment." (1974). LSU Historical Dissertations and Theses. 2674. https://digitalcommons.lsu.edu/gradschool_disstheses/2674 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

Trade-Offs Between Transport Efficiency and Power Density in Cephalopod Evolution
/. exp. Biol. 160, 93-112 (1991) 93 Printed in Great Britain © The Company of Biologists Limited 1991 INVERTEBRATE ATHLETES: TRADE-OFFS BETWEEN TRANSPORT EFFICIENCY AND POWER DENSITY IN CEPHALOPOD EVOLUTION BY R. K. O'DOR AND D. M. WEBBER Biology Department and Aquatron Laboratory, Dalhousie University, Halifax, Nova Scotia, Canada B3H 4J1 Summary Jet propulsion concentrates muscle power on a small volume of high-velocity fluid to give high thrust with low Froude efficiency. Proponents are typically escape artists with high maintenance costs. Nonetheless, oceanic squids depend primarily on jets to forage over large volumes of relatively unproductive ocean (low power density, Wm~3). A survey of locomotor performance among phyla and along an 'evolutionary continuum' of cephalopods (Nautilus, Sepia, Loligo and Illex) suggests that increasing speed and animal power density are required if animals are to compete effectively in environments of decreasing power density. Neutral buoyancy and blood oxygen reserves require unproductive volume, keeping drag high. Undulatory fins increase efficiency, but dependence on muscular hydrostats without rigid skeletal elements limits speed. Migratory oceanic squids show a remarkable range of anatomical, physiological and biochemical adaptations to sustain high speeds by maximizing power density. Muscle mitochondrial density increases 10-fold, but metabolic regulation is realigned to optimize both aerobic and anaerobic capacity. The origins of these adaptations are examined (as far as possible, and perhaps further) along the continuum leading to the most powerful invertebrates. Introduction In this Symposium, 'exercise' presumably means the production of mechanical power by animals, typically using muscle to induce movement. Muscle power comes in two forms: sustainable and burst. -

Checklist of Species Within the CCBNEP Study Area: References, Habitats, Distribution, and Abundance
Current Status and Historical Trends of the Estuarine Living Resources within the Corpus Christi Bay National Estuary Program Study Area Volume 4 of 4 Checklist of Species Within the CCBNEP Study Area: References, Habitats, Distribution, and Abundance Corpus Christi Bay National Estuary Program CCBNEP-06D • January 1996 This project has been funded in part by the United States Environmental Protection Agency under assistance agreement #CE-9963-01-2 to the Texas Natural Resource Conservation Commission. The contents of this document do not necessarily represent the views of the United States Environmental Protection Agency or the Texas Natural Resource Conservation Commission, nor do the contents of this document necessarily constitute the views or policy of the Corpus Christi Bay National Estuary Program Management Conference or its members. The information presented is intended to provide background information, including the professional opinion of the authors, for the Management Conference deliberations while drafting official policy in the Comprehensive Conservation and Management Plan (CCMP). The mention of trade names or commercial products does not in any way constitute an endorsement or recommendation for use. Volume 4 Checklist of Species within Corpus Christi Bay National Estuary Program Study Area: References, Habitats, Distribution, and Abundance John W. Tunnell, Jr. and Sandra A. Alvarado, Editors Center for Coastal Studies Texas A&M University - Corpus Christi 6300 Ocean Dr. Corpus Christi, Texas 78412 Current Status and Historical Trends of Estuarine Living Resources of the Corpus Christi Bay National Estuary Program Study Area January 1996 Policy Committee Commissioner John Baker Ms. Jane Saginaw Policy Committee Chair Policy Committee Vice-Chair Texas Natural Resource Regional Administrator, EPA Region 6 Conservation Commission Mr.