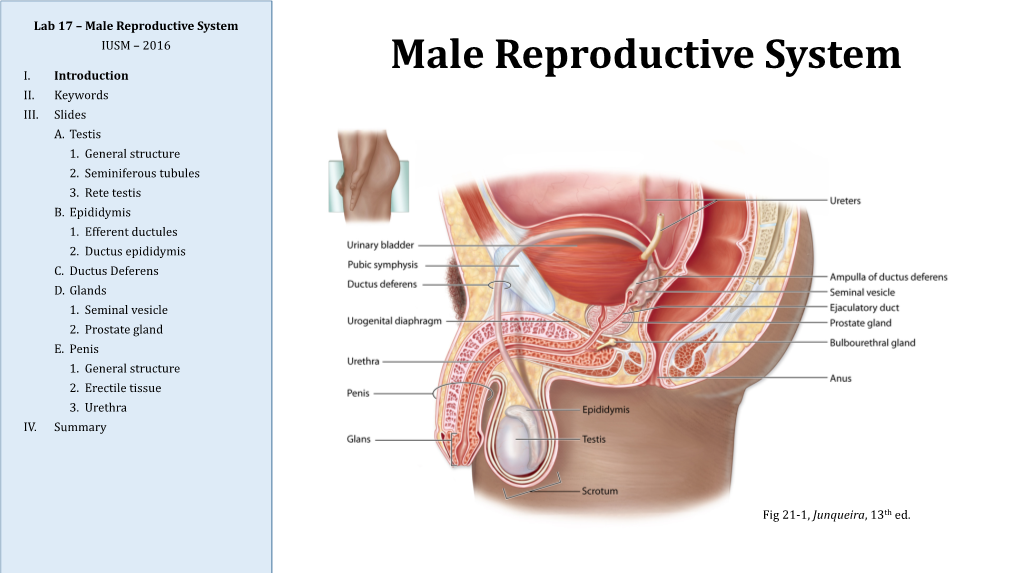
Male Reproductive System IUSM – 2016
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sonography of the Scrotum
1 Sonography of the Scrotum Chee-Wai Mak and Wen-Sheng Tzeng Department of Medical Imaging, Chi Mei Medical Center, Tainan, Taiwan Central Taiwan University of Science and Technology, Taichung, Taiwan Chung Hwa University of Medical Technology, Tainan, Taiwan Republic of China 1. Introduction Although the development of new imaging modality such as computerized tomography and magnetic resonance imaging have open a new era for medical imaging, high resolution sonography remains as the initial imaging modality of choice for evaluation of scrotal disease. Many of the disease processes, such as testicular torsion, epididymo-orchitis, and intratesticular tumor, produce the common symptom of pain at presentation, and differentiation of these conditions and disorders is important for determining the appropriate treatment. High resolution ultrasound helps in better characterize some of the intrascrotal lesions, and suggest a more specific diagnosis, resulting in more appropriate treatments and avoiding unnecessary operation for some of the diseases. 2. Imaging technique For any scrotal examination, thorough palpation of the scrotal contents and history taking should precede the sonographic examination. Patients are usually examined in the supine position with a towel draped over his thighs to support the scrotum. Warm gel should always be used because cold gel can elicit a cremasteric response resulting in thickening of the scrotal wall; hence a thorough examination is difficult to be performed. A high resolution, near-focused, linear array transducer with a frequency of 7.5 MHz or greater is often used because it provides increased resolutions of the scrotal contents. Images of both scrotum and bilateral inguinal regions are obtained in both transverse and longitudinal planes. -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -

Anatomical and Morphological Study of the Kidneys of the Breeding Emu (Dromaius Novaehollandiae)
Turkish Journal of Zoology Turk J Zool (2016) 40: 314-319 http://journals.tubitak.gov.tr/zoology/ © TÜBİTAK Research Article doi:10.3906/zoo-1506-21 Anatomical and morphological study of the kidneys of the breeding emu (Dromaius novaehollandiae) 1, 2 3 2 3 Katarzyna MICHAŁEK *, Danuta SZCZERBIŃSKA , Marta GRABOWSKA , Danuta MAJEWSKA , Maria LASZCZYŃSKA 1 Department of Physiology, Cytobiology, and Proteomics, Faculty of Biotechnology and Animal Husbandry, West Pomeranian University of Technology in Szczecin, Szczecin, Poland 2 Department of Poultry and Ornamental Bird Breeding, West Pomeranian University of Technology in Szczecin, Szczecin, Poland 3 Department of Histology and Developmental Biology, Pomeranian Medical University, Szczecin, Poland Received: 15.06.2015 Accepted/Published Online: 05.01.2016 Final Version: 07.04.2016 Abstract: We analyzed 16 kidneys obtained from 15-year-old emus, Dromaius novaehollandiae. Emus were kept at an experimental farm in Poland. The results showed that each kidney was composed of 3 parts: cranial, medial, and caudal divisions. Histological results demonstrated that the kidneys consisted of 2 zones: the cortex and the medulla. The cortex made up the majority of the kidney, while the medulla formed only a small portion of the organ. Proximal and distal tubules and 2 types of glomeruli (looped and loopless) were localized in the cortex. Each of these glomeruli was characterized by tightly arranged mesangial cells. Proximal and distal tubules had a distinctive simple low cuboidal epithelium. The luminal surface of the proximal tubules had a brush border membrane, formed by numerous microvilli. The renal medulla of emu kidneys formed irregularly positioned characteristic cones of different sizes. -

Scrotal Ultrasound
Scrotal Ultrasound Bruce R. Gilbert, MD, PhD Associate Clinical Professor of Urology & Reproductive Medicine Weill Cornell Medical College Director, Reproductive and Sexual Medicine Smith Institute For Urology North Shore LIJ Health System 1 Developmental Anatomy" Testis and Kidney Hindgut Allantois In the 3-week-old embryo the Primordial primordial germ cells in the wall of germ cells the yolk sac close to the attachment of the allantois migrate along the Heart wall of the hindgut and the dorsal Genital Ridge mesentery into the genital ridge. Yolk Sac Hindgut At 5-weeks the two excretory organs the pronephros and mesonephros systems regress Primordial Pronephric system leaving only the mesonephric duct. germ cells (regressing) Mesonephric The metanephros (adult kidney) system forms from the metanephric (regressing) diverticulum (ureteric bud) and metanephric mass of mesoderm. The ureteric bud develops as a dorsal bud of the mesonephric duct Cloaca near its insertion into the cloaca. Mesonephric Duct Mesonephric Duct Ureteric Bud Ureteric Bud Metanephric system Metanephric system 2 Developmental Anatomy" Wolffian and Mullerian DuctMesonephric Duct Under the influence of SRY, cells in the primitive sex cords differentiate into Sertoli cells forming the testis cords during week 7. Gonads Mesonephros It is at puberty that these testis cords (in Paramesonephric association with germ cells) undergo (Mullerian) Duct canalization into seminiferous tubules. Mesonephric (Wolffian) Duct At 7 weeks the indifferent embryo also has two parallel pairs of genital ducts: the Mesonephric (Wolffian) and the Paramesonephric (Mullerian) ducts. Bladder Bladder Mullerian By week 8 the developing fetal testis tubercle produces at least two hormones: Metanephros 1. A glycoprotein (MIS) produced by the Ureter Uterovaginal fetal Sertoli cells (in response to SRY) primordium Rectum which suppresses unilateral development of the Paramesonephric (Mullerian) duct 2. -

Male Reproductive System
MALE REPRODUCTIVE SYSTEM DR RAJARSHI ASH M.B.B.S.(CAL); D.O.(EYE) ; M.D.-PGT(2ND YEAR) DEPARTMENT OF PHYSIOLOGY CALCUTTA NATIONAL MEDICAL COLLEGE PARTS OF MALE REPRODUCTIVE SYSTEM A. Gonads – Two ovoid testes present in scrotal sac, out side the abdominal cavity B. Accessory sex organs - epididymis, vas deferens, seminal vesicles, ejaculatory ducts, prostate gland and bulbo-urethral glands C. External genitalia – penis and scrotum ANATOMY OF MALE INTERNAL GENITALIA AND ACCESSORY SEX ORGANS SEMINIFEROUS TUBULE Two principal cell types in seminiferous tubule Sertoli cell Germ cell INTERACTION BETWEEN SERTOLI CELLS AND SPERM BLOOD- TESTIS BARRIER • Blood – testis barrier protects germ cells in seminiferous tubules from harmful elements in blood. • The blood- testis barrier prevents entry of antigenic substances from the developing germ cells into circulation. • High local concentration of androgen, inositol, glutamic acid, aspartic acid can be maintained in the lumen of seminiferous tubule without difficulty. • Blood- testis barrier maintains higher osmolality of luminal content of seminiferous tubules. FUNCTIONS OF SERTOLI CELLS 1.Germ cell development 2.Phagocytosis 3.Nourishment and growth of spermatids 4.Formation of tubular fluid 5.Support spermiation 6.FSH and testosterone sensitivity 7.Endocrine functions of sertoli cells i)Inhibin ii)Activin iii)Follistatin iv)MIS v)Estrogen 8.Sertoli cell secretes ‘Androgen binding protein’(ABP) and H-Y antigen. 9.Sertoli cell contributes formation of blood testis barrier. LEYDIG CELL • Leydig cells are present near the capillaries in the interstitial space between seminiferous tubules. • They are rich in mitochondria & endoplasmic reticulum. • Leydig cells secrete testosterone,DHEA & Androstenedione. • The activity of leydig cell is different in different phases of life. -

Morphology of the Male Reproductive Tract in the Water Scavenger Beetle Tropisternus Collaris Fabricius, 1775 (Coleoptera: Hydrophilidae)
Revista Brasileira de Entomologia 65(2):e20210012, 2021 Morphology of the male reproductive tract in the water scavenger beetle Tropisternus collaris Fabricius, 1775 (Coleoptera: Hydrophilidae) Vinícius Albano Araújo1* , Igor Luiz Araújo Munhoz2, José Eduardo Serrão3 1Universidade Federal do Rio de Janeiro, Instituto de Biodiversidade e Sustentabilidade (NUPEM), Macaé, RJ, Brasil. 2Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. 3Universidade Federal de Viçosa, Departamento de Biologia Geral, Viçosa, MG, Brasil. ARTICLE INFO ABSTRACT Article history: Members of the Hydrophilidae, one of the largest families of aquatic insects, are potential models for the Received 07 February 2021 biomonitoring of freshwater habitats and global climate change. In this study, we describe the morphology of Accepted 19 April 2021 the male reproductive tract in the water scavenger beetle Tropisternus collaris. The reproductive tract in sexually Available online 12 May 2021 mature males comprised a pair of testes, each with at least 30 follicles, vasa efferentia, vasa deferentia, seminal Associate Editor: Marcela Monné vesicles, two pairs of accessory glands (a bean-shaped pair and a tubular pair with a forked end), and an ejaculatory duct. Characters such as the number of testicular follicles and accessory glands, as well as their shape, origin, and type of secretion, differ between Coleoptera taxa and have potential to help elucidate reproductive strategies and Keywords: the evolutionary history of the group. Accessory glands Hydrophilid Polyphaga Reproductive system Introduction Coleoptera is the most diverse group of insects in the current fauna, The evolutionary history of Coleoptera diversity (Lawrence et al., with about 400,000 described species and still thousands of new species 1995; Lawrence, 2016) has been grounded in phylogenies with waiting to be discovered (Slipinski et al., 2011; Kundrata et al., 2019). -

Ultrasonography of the Scrotum in Adults
University of Massachusetts Medical School eScholarship@UMMS Radiology Publications and Presentations Radiology 2016-07-01 Ultrasonography of the scrotum in adults Anna L. Kuhn University of Massachusetts Medical School Et al. Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/radiology_pubs Part of the Male Urogenital Diseases Commons, Radiology Commons, Reproductive and Urinary Physiology Commons, Urogenital System Commons, and the Urology Commons Repository Citation Kuhn AL, Scortegagna E, Nowitzki KM, Kim YH. (2016). Ultrasonography of the scrotum in adults. Radiology Publications and Presentations. https://doi.org/10.14366/usg.15075. Retrieved from https://escholarship.umassmed.edu/radiology_pubs/173 Creative Commons License This work is licensed under a Creative Commons Attribution-Noncommercial 3.0 License This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in Radiology Publications and Presentations by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. Ultrasonography of the scrotum in adults Anna L. Kühn, Eduardo Scortegagna, Kristina M. Nowitzki, Young H. Kim Department of Radiology, UMass Memorial Medical Center, University of Massachusetts Medical Center, Worcester, MA, USA REVIEW ARTICLE Ultrasonography is the ideal noninvasive imaging modality for evaluation of scrotal http://dx.doi.org/10.14366/usg.15075 abnormalities. It is capable of differentiating the most important etiologies of acute scrotal pain pISSN: 2288-5919 • eISSN: 2288-5943 and swelling, including epididymitis and testicular torsion, and is the imaging modality of choice Ultrasonography 2016;35:180-197 in acute scrotal trauma. In patients presenting with palpable abnormality or scrotal swelling, ultrasonography can detect, locate, and characterize both intratesticular and extratesticular masses and other abnormalities. -

PROSTATE and TESTIS PATHOLOGY “A Coin Has Two Sides”, the Duality of Male Pathology
7/12/2017 PROSTATE AND TESTIS PATHOLOGY “A Coin Has Two Sides”, The Duality Of Male Pathology • Jaime Furman, M.D. • Pathology Reference Laboratory San Antonio. • Clinical Assistant Professor Departments of Pathology and Urology, UT Health San Antonio. Source: http://themoderngoddess.com/blog/spring‐equinox‐balance‐in‐motion/ I am Colombian and speak English with a Spanish accent! o Shannon Alporta o Lindsey Sinn o Joe Nosito o Megan Bindseil o Kandace Michael o Savannah McDonald Source: http://www.taringa.net/posts/humor/7967911/Sindrome‐de‐la‐ Tiza.html 1 7/12/2017 The Prostate Axial view Base Apex Middle Apex Sagittal view Reference: Vikas Kundra, M.D., Ph.D. , Surena F. Matin, M.D. , Deborah A. Kuban, M.Dhttps://clinicalgate.com/prostate‐cancer‐4/ Ultrasound‐guided biopsy following a specified grid pattern of biopsies remains the standard of care. This approach misses 21% to 28% of prostate cancers. JAMA. 2017;317(24):2532‐2542. http://www.nature.com/nrurol/journal/v10/n12/abs/nrurol.2013.195.html Prostate Pathology Inflammation / granulomas Categories Adenosis, radiation, atrophy seminal vesicle Biopsy Benign TURP HGPIN Unsuspected carcinoma is seen in 12% of Atypical IHC TURP cases. glands Prostatectomy Subtype, Gleason, Malignant fat invasion, vascular invasion Other malignancies: sarcomas, lymphomas Benign Prostate Remember Malignant Glands Lack Basal Glands Cells Basal cells Secretory cells Stroma 2 7/12/2017 Benign Prostatic Lesions Atrophy Corpora amylacea (secretions) Seminal Vesicle Acute inflammation GMS Basal cell hyperplasia Basal cell hyperplasia Granulomas (BPH) (BPH) coccidiomycosis Mimics of Prostate Carcinoma Atrophy. Benign Carcinoma with atrophic features Prostate Carcinoma 1. Prostate cancer is the most common, noncutaneous cancer in men in the United States. -

The Male Body
Fact Sheet The Male Body What is the male What is the epididymis? reproductive system? The epididymis is a thin highly coiled tube (duct) A man’s fertility and sexual characteristics depend that lies at the back of each testis and connects on the normal functioning of the male reproductive the seminiferous tubules in the testis to another system. A number of individual organs act single tube called the vas deferens. together to make up the male reproductive 1 system; some are visible, such as the penis and the 6 scrotum, whereas some are hidden within the body. The brain also has an important role in controlling 7 12 reproductive function. 2 8 1 11 What are the testes? 3 6 The testes (testis: singular) are a pair of egg 9 7 12 shaped glands that sit in the scrotum next to the 2 8 base of the penis on the outside of the body. In 4 10 11 adult men, each testis is normally between 15 and 3 35 mL in volume. The testes are needed for the 5 male reproductive system to function normally. 9 The testes have two related but separate roles: 4 10 • to make sperm 5 1 Bladder • to make testosterone. 2 Vas deferens The testes develop inside the abdomen in the 3 Urethra male fetus and then move down (descend) into the scrotum before or just after birth. The descent 4 Penis of the testes is important for fertility as a cooler 5 Scrotum temperature is needed to make sperm and for 16 BladderSeminal vesicle normal testicular function. -

Anatomy and Physiology Male Reproductive System References
DEWI PUSPITA ANATOMY AND PHYSIOLOGY MALE REPRODUCTIVE SYSTEM REFERENCES . Tortora and Derrickson, 2006, Principles of Anatomy and Physiology, 11th edition, John Wiley and Sons Inc. Medical Embryology Langeman, pdf. Moore and Persaud, The Developing Human (clinically oriented Embryologi), 8th edition, Saunders, Elsevier, . Van de Graff, Human anatomy, 6th ed, Mcgraw Hill, 2001,pdf . Van de Graff& Rhees,Shaum_s outline of human anatomy and physiology, Mcgraw Hill, 2001, pdf. WHAT IS REPRODUCTION SYSTEM? . Unlike other body systems, the reproductive system is not essential for the survival of the individual; it is, however, required for the survival of the species. The RS does not become functional until it is “turned on” at puberty by the actions of sex hormones sets the reproductive system apart. The male and female reproductive systems complement each other in their common purpose of producing offspring. THE TOPIC : . 1. Gamet Formation . 2. Primary and Secondary sex organ . 3. Male Reproductive system . 4. Female Reproductive system . 5. Female Hormonal Cycle GAMET FORMATION . Gamet or sex cells are the functional reproductive cells . Contain of haploid (23 chromosomes-single) . Fertilizationdiploid (23 paired chromosomes) . One out of the 23 pairs chromosomes is the determine sex sex chromosome X or Y . XXfemale, XYmale Gametogenesis Oocytes Gameto Spermatozoa genesis XY XX XX/XY MALE OR FEMALE....? Male Reproductive system . Introduction to the Male Reproductive System . Scrotum . Testes . Spermatic Ducts, Accessory Reproductive Glands,and the Urethra . Penis . Mechanisms of Erection, Emission, and Ejaculation The urogenital system . Functionally the urogenital system can be divided into two entirely different components: the urinary system and the genital system. -

Diagnosis and Management of Infertility Due to Ejaculatory Duct Obstruction: Summary Evidence ______
Vol. 47 (4): 868-881, July - August, 2021 doi: 10.1590/S1677-5538.IBJU.2020.0536 EXPERT OPINION Diagnosis and management of infertility due to ejaculatory duct obstruction: summary evidence _______________________________________________ Arnold Peter Paul Achermann 1, 2, 3, Sandro C. Esteves 1, 2 1 Departmento de Cirurgia (Disciplina de Urologia), Universidade Estadual de Campinas - UNICAMP, Campinas, SP, Brasil; 2 ANDROFERT, Clínica de Andrologia e Reprodução Humana, Centro de Referência para Reprodução Masculina, Campinas, SP, Brasil; 3 Urocore - Centro de Urologia e Fisioterapia Pélvica, Londrina, PR, Brasil INTRODUCTION tion or perineal pain exacerbated by ejaculation and hematospermia (3). These observations highlight the Infertility, defined as the failure to conceive variability in clinical presentations, thus making a after one year of unprotected regular sexual inter- comprehensive workup paramount. course, affects approximately 15% of couples worl- EDO is of particular interest for reproduc- dwide (1). In about 50% of these couples, the male tive urologists as it is a potentially correctable factor, alone or combined with a female factor, is cause of male infertility. Spermatogenesis is well- contributory to the problem (2). Among the several -preserved in men with EDO owing to its obstruc- male infertility conditions, ejaculatory duct obstruc- tive nature, thus making it appealing to relieve the tion (EDO) stands as an uncommon causative factor. obstruction and allow these men the opportunity However, the correct diagnosis and treatment may to impregnate their partners naturally. This review help the affected men to impregnate their partners aims to update practicing urologists on the current naturally due to its treatable nature. methods for diagnosis and management of EDO.