A61p25/00 (2006.01) A61p29/00 (2006.01)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Final Program
IPA-0716-539 Final Program #IPASanFran2016 ABOUT IPA Founded in 1982, the International Psychogeriatric Association (IPA) is a unique and diverse professional healthcare community promoting better geriatric mental health – across disciplines, across borders, and across geriatric issues. Psychiatrists, Scientists, Neurologists, Geriatricians, Primary Care Physicians, Epidemiologists, Nurses, Psychologists, Occupational Therapists, Social Workers and many other healthcare profession- als come to the IPA community from all over the world to discuss, learn, share and research information about behavioral and biological aspects of geriatric mental health. IPA Member Benefits • Subscription to International Psychogeriatrics, IPA’s multidisciplinary peer-reviewed journal published monthly • Subscription to the IPA Bulletin, IPA’s membership newsletter • Reduced rates and access to IPA congress and meetings • IPA Online – the IPA website – including the IPA Member Area with special features including access to fellow members and The IPA Complete Guide to Behavioral and Psychological Symptoms of Dementia (BPSD) • Participation in IPA Member Forums, IPA’s in-person and online groups which enable members to meet, communicate, and collaborate with IPA colleagues around the world. Groups include: Regional Initiative Forums (groups by geographic areas); Professional Discipline Forums (groups such as nurses, psychologists, primary care physicians, social workers, and occupational therapists); and Shared Interest Forums (interest groups including long term care, young onset dementia, testamentary capacity and others) IPA Board of Directors OFFICERS DIRECTORS EX OFFICIO Raimundo Mateos, President, Spain Sabine Bährer-Kohler, Switzerland Manabu Ikeda, Japan Mary Sano, President-Elect, United States David Conn, Canada IPA SECRETARIAT Huali Wang, Secretary, PR China Brian Draper, Australia Kate Filipiak, CAE, Executive Director Constantine G. -

Dementia: Types, Stages and Sleep Disturbances
DEMENTIA: TYPES, STAGES AND SLEEP DISTURBANCES Ericka L. Crouse, PharmD, BCPP, BCGP Associate Professor VCU School of Pharmacy Disclosures Dr. Crouse has nothing to disclose Objectives 1. Compare and contrast different types of dementia 2. Differentiate between stages of dementia and role of pharmacotherapy in each stage 3. List pros and cons of medications to treat sleep disturbances in dementia Pre-Assessment Question Which type of dementia is characterized by visual hallucinations especially of small animals and objects? A. Alzheimer’s B. Vascular C. Lewy-body D. Fronto-temporal Pre-Assessment Question Memantine is FDA indicated for which stage of Alzheimer’s disease? A. Mild cognitive impairment B. Mild/moderate C. Moderate/severe D. Only in combination with donepezil for mild/moderate Pre-Assessment Question Rapid-eye movement (REM) sleep behavior disorder is associated with which type of dementia? A. Alzheimer’s B. Vascular C. Lewy-body D. Fronto-temporal Pre-Assessment Question Which of the following is a risk associated with use of zolpidem in elderly patients? A. Falls with injury B. Decreased appetite C. Cognitive Impairment D. Anticholinergic effects Title TYPES OF DEMENTIA Change in Terminology DSM-IV DSM-5 • Dementia • Neurocognitive Disorder DSM = Diagnostic and Statistical Manual of Mental Disorders DSM-5 Am Psychiatric Assoc 2013 DSM-5 Criteria Evidence of a significant decline in cognitive function from previous level in ≥ 1 cognitive domain - Complex attention, executive function, learning and memory, language, perceptual-motor -

Download Final Program
September 20 – 23 2016 FINAL PROGRAM Bringing the Parkinson’s Community Together! Welcome Letters ................................................... 1-3 Committee Members ................................................ 4 General Information ............................................... 5-8 Wellness Way ...................................................11-14 WPC Theater .........................................................15 WPC Art Walk ........................................................17 TABLE OF WPC Awards .........................................................18 CONTENTS Social Program and Special Events ............................... 20 Travel Grants and Travel Grants Supporters ............22-23 Continuing Education ............................................... 24 Program-at-a-Glance .......................................... 26-27 Congress Program .............................................29-56 Posters and Poster Tours .................................... 57-95 Exhibition ........................................................ 96-115 PD Glossary .................................................. 118-126 Faculty List ......................................................... 127 Oregon Convention Center ....................................... 128 Portland Map .......................................................129 Organizational Partners ......................................... 130 Acknowledgements ...............................................131 The purpose of the World Parkinson Congresses is to create -

W O 2016/201373 A1 15 December 2016 (15.12.2016) W I PO I P C T
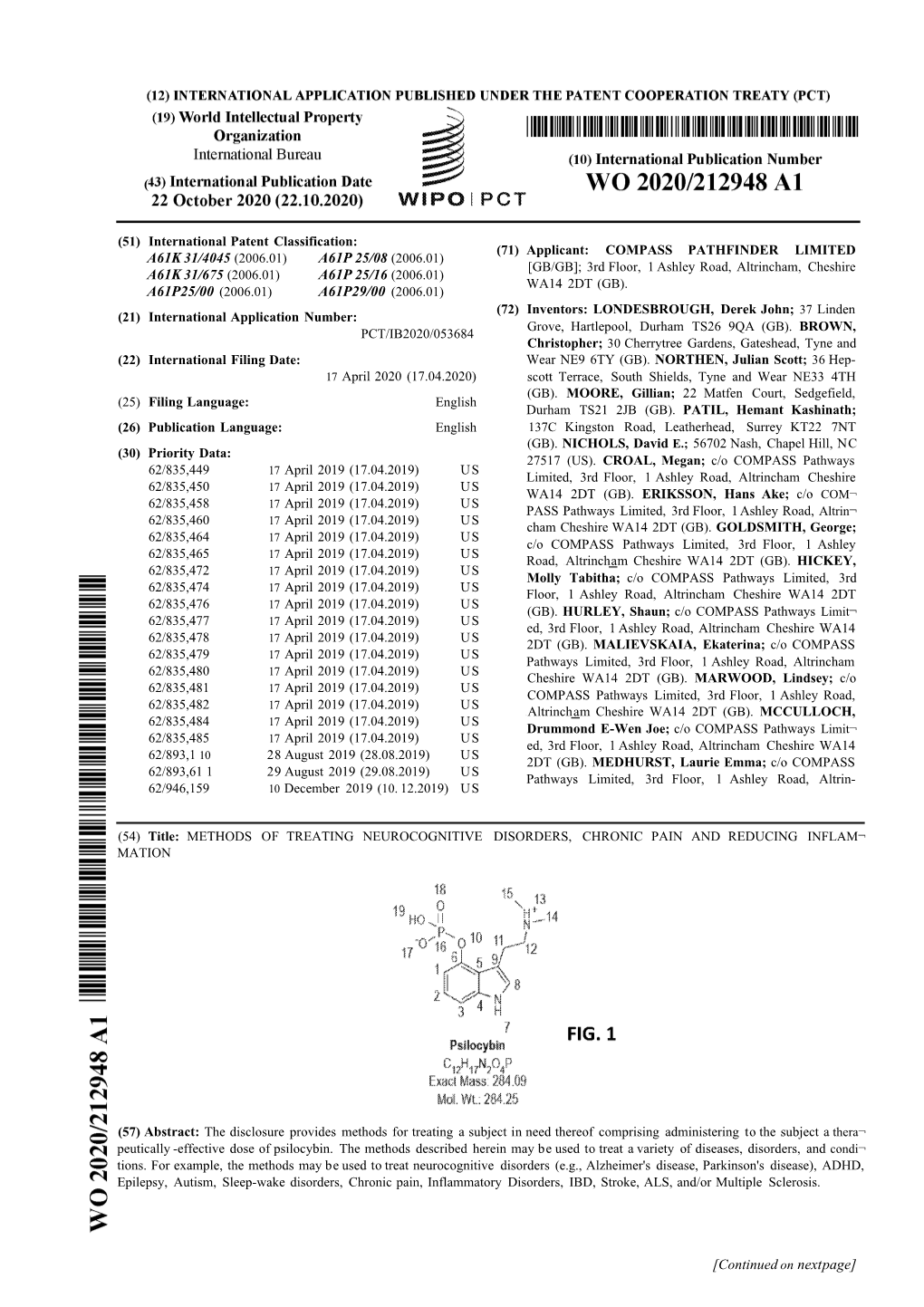
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date W O 2016/201373 A1 15 December 2016 (15.12.2016) W I PO I P C T (51) International Patent Classification: (74) Agents: QUEIROZ DE OLIVEIRA, Pierre et al.; Pepper A61K31/17(2006.01) A61P 25/00 (2006.01) Hamilton LLP, 500 Grant Street, Suite 5000, Pittsburgh, A61K 31/415 (2006.01) PA 15219-2507 (US). (21) International Application Number: (81) Designated States (unless otherwise indicated,for every PCT/US2016/037090 kind of national protection available): AE, AG, AL, AM, .) . AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (22) International Filng Date: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, 10 June 2016 (10.06.2016) DO, DZ, EC, EE, EG, ES, Fl, GB, GD, GE, GH, GM, GT, (25) Filing Language: English HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (26) Publication Language: English MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (30) Priority Data: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 62/174,983 12 June 2015 (12.06.2015) US SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 62/194,084 17 July 2015 (17.07.2015) US TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

GPCR/G Protein
Inhibitors, Agonists, Screening Libraries www.MedChemExpress.com GPCR/G Protein G Protein Coupled Receptors (GPCRs) perceive many extracellular signals and transduce them to heterotrimeric G proteins, which further transduce these signals intracellular to appropriate downstream effectors and thereby play an important role in various signaling pathways. G proteins are specialized proteins with the ability to bind the nucleotides guanosine triphosphate (GTP) and guanosine diphosphate (GDP). In unstimulated cells, the state of G alpha is defined by its interaction with GDP, G beta-gamma, and a GPCR. Upon receptor stimulation by a ligand, G alpha dissociates from the receptor and G beta-gamma, and GTP is exchanged for the bound GDP, which leads to G alpha activation. G alpha then goes on to activate other molecules in the cell. These effects include activating the MAPK and PI3K pathways, as well as inhibition of the Na+/H+ exchanger in the plasma membrane, and the lowering of intracellular Ca2+ levels. Most human GPCRs can be grouped into five main families named; Glutamate, Rhodopsin, Adhesion, Frizzled/Taste2, and Secretin, forming the GRAFS classification system. A series of studies showed that aberrant GPCR Signaling including those for GPCR-PCa, PSGR2, CaSR, GPR30, and GPR39 are associated with tumorigenesis or metastasis, thus interfering with these receptors and their downstream targets might provide an opportunity for the development of new strategies for cancer diagnosis, prevention and treatment. At present, modulators of GPCRs form a key area for the pharmaceutical industry, representing approximately 27% of all FDA-approved drugs. References: [1] Moreira IS. Biochim Biophys Acta. 2014 Jan;1840(1):16-33. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -
Full Application
PI: Wighton, Paul Title: Unbiased longitudinal neuromorphometry for clinical decision support Received: 09/04/2018 FOA: PAS18-188 Council: 01/2019 Clinical Trial:Optional Competition ID: FORMS-E FOA Title: Advancing Research on Alzheimer's Disease (AD) and Alzheimer's- Disease-Related Dementias (ADRD) (R41/R42 Clinical Trial Optional) 1 R42 AG062026-01A1 Dual: blank Accession Number: 4209265 IPF: 10032164 Organization: CORTICOMETRICS, LLC Former Number: blank Department: blank IRG/SRG: ZRG1 SBIB-T (10)B AIDS: N Expedited: N Subtotal Direct Costs Animals: N New Investigator: blank (excludes consortium F&A) Humans: N Early Stage Investigator: blank Year 1: Clinical Trial: N Year 2: Current HS Code: 10 Year 3: HESC: N Senior/Key Personnel: Organization: Role Category: Paul Wighton CORTICOMETRICS, LLC PD/PI Bruce Fischl Massachusetts General Hospital (The MPI General Hospital Corp) STEVEN ARNOLD MASSACHUSETTS GENERAL Co-Investigator HOSPITAL Otto Rapalino Massachusetts General Hospital (The Co-Investigator General Hospital Corp) Brian Edlow Massachusetts General Hospital (The Co-Investigator General Hospital Corp) Martin Reuter Massachusetts General Hospital (The Co-Investigator General Hospital Corp) STEVEN STUFFLEBEAM Massachusetts General Hospital (The Co-Investigator General Hospital Corp) Nicholas Schmansky CORTICOMETRICS, LLC Other Professional-CEO, Software Engineer OMB Number: 4040-0001 Expiration Date: 10/31/2019 APPLICATION FOR FEDERAL ASSISTANCE 3. DATE RECEIVED BY STATE State Application Identifier SF 424 (R&R) blank blank 1. TYPE -

Synthesis of Novel Aporphine-Inspired Neuroreceptor Ligands
City University of New York (CUNY) CUNY Academic Works All Dissertations, Theses, and Capstone Projects Dissertations, Theses, and Capstone Projects 2-2016 Synthesis of Novel Aporphine-Inspired Neuroreceptor Ligands Nirav R. Kapadia Graduate Center, City University of New York How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/gc_etds/792 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] SYNTHESIS OF NOVEL APORPHINE-INSPIRED NEURORECEPTOR LIGANDS by Nirav R Kapadia A dissertation submitted to the Graduate Faculty in Chemistry in partial fulfillment of the requirements for the degree of Doctor of Philosophy, The City University of New York. 2016 © 2016 NIRAV R KAPADIA All Rights Reserved ii This manuscript has been read and accepted by the Graduate Faculty in Chemistry in satisfaction of the dissertation requirement for the degree of Doctor of Philosophy ____________________ _________________________ Date Dr. Wayne Harding Chair of Examining Committee ____________________ __________________________ Date Dr. Brian Gibney Executive Officer Dr. Adam Profit Dr. Shengping Zheng Dr. Wayne Harding Supervisory Committee THE CITY UNIVERSITY OF NEW YORK iii ABSTRACT Synthesis of Novel Aporphine-Inspired Neuroreceptor Ligands by Nirav R Kapadia Advisor: Dr. Wayne Harding Aporphines are a group of tetracyclic alkaloids that belong to the ubiquitous tetrahydroisoquinoline family. The aporphine template is known to be associated with a range of biological activities. Aporphines have been explored as antioxidants, anti-tuberculosis, antimicrobial and anticancer agents. Within the Central Nervous Systems (CNS), aporphine alkaloids are known to possess high affinity for several clinically valuable targets including dopamine receptors (predominantly D1 and D2), serotonin receptors (5-HT1A and 5-HT7) and α adrenergic receptors. -

Treatment of Chronic Pain by Medical Approaches the AMERICAN ACADEMY of PAIN MEDICINE Textbook on Patient Management Treatment of Chronic Pain by Medical Approaches
the AMERICAN ACADEMY of PAIN MEDICINE Timothy R. Deer Vitaly Gordin Editor-in-Chief Associate Editor Michael S. Leong Associate Editor-in-Chief Treatment of Chronic Pain by Medical Approaches the AMERICAN ACADEMY of PAIN MEDICINE Textbook on Patient Management Treatment of Chronic Pain by Medical Approaches Timothy R. Deer Editor-in-chief Michael S. Leong Associate Editor-in-chief Vitaly Gordin Associate Editor Treatment of Chronic Pain by Medical Approaches the AMERICAN ACADEMY of PAIN MEDICINE Textbook on Patient Management Editor-in-chief Associate Editor-in-chief Timothy R. Deer, M.D. Michael S. Leong, M.D. President and CEO Clinic Chief The Center for Pain Relief Stanford Pain Medicine Center Clinical Professor of Anesthesiology Redwood City, CA, USA West Virginia University School of Medicine Clinical Associate Professor Charleston , WV, USA Department of Anesthesiology Stanford University School of Medicine Associate Editor Stanford , CA , USA Vitaly Gordin, M.D. Associate Professor Associate Vice Chair of Pain Management Department of Anesthesiology Pennsylvania State University College of Medicine Director Pain Medicine Milton S. Hershey Medical Center Hershey , PA , USA ISBN 978-1-4939-1817-1 ISBN 978-1-4939-1818-8 (eBook) DOI 10.1007/978-1-4939-1818-8 Springer New York Heidelberg Dordrecht London Library of Congress Control Number: 2014952895 © American Academy of Pain Medicine 2015 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfi lms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. -

WO 2020/212952 A1 22 October 2020 (22.10.2020)
(12) INTERNATIONAL APPLICATION PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization iii iiiii min linn ill II Minim mill ill m International Bureau (10) International Publication Number (43) International Publication Date WO 2020/212952 A1 22 October 2020 (22.10.2020) (51) International Patent Classification: 62/946, 159 10 December 2019 (10. 12.2019) U S A61K 31/675 (2006.01) A61P 25/30 (2006.01) (71) Applicant: COMPASS PATHFINDER LIMITED A61K 31/4045 (2006.01) A61P 25/00 (2006.01) [GB/GB]; 3rd Floor, 1 Ashley Road, Altrincham, Cheshire A61P1/00 (2006.01) A61P 25/06 (2006.01) WA14 2DT (GB). A61P 25/16 (2006.01) A61P25/22 (2006.01) (72) Inventors: LONDESBROUGH, Derek John; 37 Linden (21) International Application Number: Grove, Hartlepool, Durham TS26 9QA (GB). BROWN, PCT/IB2020/053688 Christopher; 30 Cherrytree Gardens, Gateshead, Tyne and (22) International Filing Date: Wear NE9 6TY (GB). NORTHEN, Julian Scott; 36 Hep- 17 April 2020 (17.04.2020) scott Terrace, South Shields, Tyne and Wear NE33 4TH (GB). MOORE, Gillian; 22 Matfen Court, Sedgefield, (25) Filing Language: English Durham TS21 2JB (GB). PATIL, Hemant Kashinath; (26) Publication Language: English 137C Kingston Road, Leatherhead, Surrey KT22 7NT (GB). NICHOLS, David E.; 56702 Nash, Chapel Hill, N C (30) Priority Data: 27517 (US). CROAL, Megan; c/o COMPASS Pathways 62/835,449 17 April 2019 (17.04.2019) U S Limited, 3rd Floor, 1 Ashley Road, Altrincham Cheshire 62/835,450 17 April 2019 (17.04.2019) U S WA14 2DT (GB). ERIKSSON, Hans Ake; c/o COM¬ 62/835,458 17 April 2019 (17.04.2019) U S PASS Pathways Limited, 3rd Floor, 1 Ashley Road, Altrin¬ 62/835,460 17 April 2019 (17.04.2019) U S cham Cheshire WA14 2DT (GB). -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

A Abacavir Abacavirum Abakaviiri Abagovomab Abagovomabum
A abacavir abacavirum abakaviiri abagovomab abagovomabum abagovomabi abamectin abamectinum abamektiini abametapir abametapirum abametapiiri abanoquil abanoquilum abanokiili abaperidone abaperidonum abaperidoni abarelix abarelixum abareliksi abatacept abataceptum abatasepti abciximab abciximabum absiksimabi abecarnil abecarnilum abekarniili abediterol abediterolum abediteroli abetimus abetimusum abetimuusi abexinostat abexinostatum abeksinostaatti abicipar pegol abiciparum pegolum abisipaaripegoli abiraterone abirateronum abirateroni abitesartan abitesartanum abitesartaani ablukast ablukastum ablukasti abrilumab abrilumabum abrilumabi abrineurin abrineurinum abrineuriini abunidazol abunidazolum abunidatsoli acadesine acadesinum akadesiini acamprosate acamprosatum akamprosaatti acarbose acarbosum akarboosi acebrochol acebrocholum asebrokoli aceburic acid acidum aceburicum asebuurihappo acebutolol acebutololum asebutololi acecainide acecainidum asekainidi acecarbromal acecarbromalum asekarbromaali aceclidine aceclidinum aseklidiini aceclofenac aceclofenacum aseklofenaakki acedapsone acedapsonum asedapsoni acediasulfone sodium acediasulfonum natricum asediasulfoninatrium acefluranol acefluranolum asefluranoli acefurtiamine acefurtiaminum asefurtiamiini acefylline clofibrol acefyllinum clofibrolum asefylliiniklofibroli acefylline piperazine acefyllinum piperazinum asefylliinipiperatsiini aceglatone aceglatonum aseglatoni aceglutamide aceglutamidum aseglutamidi acemannan acemannanum asemannaani acemetacin acemetacinum asemetasiini aceneuramic