ASX: KZA) Re-Initiation of Coverage – Tuesday 3 April 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects
International Journal of Molecular Sciences Review PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects Rosalin Mishra , Hima Patel, Samar Alanazi , Mary Kate Kilroy and Joan T. Garrett * Department of Pharmaceutical Sciences, College of Pharmacy, University of Cincinnati, Cincinnati, OH 45267-0514, USA; [email protected] (R.M.); [email protected] (H.P.); [email protected] (S.A.); [email protected] (M.K.K.) * Correspondence: [email protected]; Tel.: +1-513-558-0741; Fax: +1-513-558-4372 Abstract: The phospatidylinositol-3 kinase (PI3K) pathway is a crucial intracellular signaling pathway which is mutated or amplified in a wide variety of cancers including breast, gastric, ovarian, colorectal, prostate, glioblastoma and endometrial cancers. PI3K signaling plays an important role in cancer cell survival, angiogenesis and metastasis, making it a promising therapeutic target. There are several ongoing and completed clinical trials involving PI3K inhibitors (pan, isoform-specific and dual PI3K/mTOR) with the goal to find efficient PI3K inhibitors that could overcome resistance to current therapies. This review focuses on the current landscape of various PI3K inhibitors either as monotherapy or in combination therapies and the treatment outcomes involved in various phases of clinical trials in different cancer types. There is a discussion of the drug-related toxicities, challenges associated with these PI3K inhibitors and the adverse events leading to treatment failure. In addition, novel PI3K drugs that have potential to be translated in the clinic are highlighted. Keywords: cancer; PIK3CA; resistance; PI3K inhibitors Citation: Mishra, R.; Patel, H.; Alanazi, S.; Kilroy, M.K.; Garrett, J.T. -

Final Program
IPA-0716-539 Final Program #IPASanFran2016 ABOUT IPA Founded in 1982, the International Psychogeriatric Association (IPA) is a unique and diverse professional healthcare community promoting better geriatric mental health – across disciplines, across borders, and across geriatric issues. Psychiatrists, Scientists, Neurologists, Geriatricians, Primary Care Physicians, Epidemiologists, Nurses, Psychologists, Occupational Therapists, Social Workers and many other healthcare profession- als come to the IPA community from all over the world to discuss, learn, share and research information about behavioral and biological aspects of geriatric mental health. IPA Member Benefits • Subscription to International Psychogeriatrics, IPA’s multidisciplinary peer-reviewed journal published monthly • Subscription to the IPA Bulletin, IPA’s membership newsletter • Reduced rates and access to IPA congress and meetings • IPA Online – the IPA website – including the IPA Member Area with special features including access to fellow members and The IPA Complete Guide to Behavioral and Psychological Symptoms of Dementia (BPSD) • Participation in IPA Member Forums, IPA’s in-person and online groups which enable members to meet, communicate, and collaborate with IPA colleagues around the world. Groups include: Regional Initiative Forums (groups by geographic areas); Professional Discipline Forums (groups such as nurses, psychologists, primary care physicians, social workers, and occupational therapists); and Shared Interest Forums (interest groups including long term care, young onset dementia, testamentary capacity and others) IPA Board of Directors OFFICERS DIRECTORS EX OFFICIO Raimundo Mateos, President, Spain Sabine Bährer-Kohler, Switzerland Manabu Ikeda, Japan Mary Sano, President-Elect, United States David Conn, Canada IPA SECRETARIAT Huali Wang, Secretary, PR China Brian Draper, Australia Kate Filipiak, CAE, Executive Director Constantine G. -

Dementia: Types, Stages and Sleep Disturbances
DEMENTIA: TYPES, STAGES AND SLEEP DISTURBANCES Ericka L. Crouse, PharmD, BCPP, BCGP Associate Professor VCU School of Pharmacy Disclosures Dr. Crouse has nothing to disclose Objectives 1. Compare and contrast different types of dementia 2. Differentiate between stages of dementia and role of pharmacotherapy in each stage 3. List pros and cons of medications to treat sleep disturbances in dementia Pre-Assessment Question Which type of dementia is characterized by visual hallucinations especially of small animals and objects? A. Alzheimer’s B. Vascular C. Lewy-body D. Fronto-temporal Pre-Assessment Question Memantine is FDA indicated for which stage of Alzheimer’s disease? A. Mild cognitive impairment B. Mild/moderate C. Moderate/severe D. Only in combination with donepezil for mild/moderate Pre-Assessment Question Rapid-eye movement (REM) sleep behavior disorder is associated with which type of dementia? A. Alzheimer’s B. Vascular C. Lewy-body D. Fronto-temporal Pre-Assessment Question Which of the following is a risk associated with use of zolpidem in elderly patients? A. Falls with injury B. Decreased appetite C. Cognitive Impairment D. Anticholinergic effects Title TYPES OF DEMENTIA Change in Terminology DSM-IV DSM-5 • Dementia • Neurocognitive Disorder DSM = Diagnostic and Statistical Manual of Mental Disorders DSM-5 Am Psychiatric Assoc 2013 DSM-5 Criteria Evidence of a significant decline in cognitive function from previous level in ≥ 1 cognitive domain - Complex attention, executive function, learning and memory, language, perceptual-motor -

Download Final Program
September 20 – 23 2016 FINAL PROGRAM Bringing the Parkinson’s Community Together! Welcome Letters ................................................... 1-3 Committee Members ................................................ 4 General Information ............................................... 5-8 Wellness Way ...................................................11-14 WPC Theater .........................................................15 WPC Art Walk ........................................................17 TABLE OF WPC Awards .........................................................18 CONTENTS Social Program and Special Events ............................... 20 Travel Grants and Travel Grants Supporters ............22-23 Continuing Education ............................................... 24 Program-at-a-Glance .......................................... 26-27 Congress Program .............................................29-56 Posters and Poster Tours .................................... 57-95 Exhibition ........................................................ 96-115 PD Glossary .................................................. 118-126 Faculty List ......................................................... 127 Oregon Convention Center ....................................... 128 Portland Map .......................................................129 Organizational Partners ......................................... 130 Acknowledgements ...............................................131 The purpose of the World Parkinson Congresses is to create -

A Report to the President U.S
A Report to the President U.S. Department of the Interior 2017-2021 REPORT TO THE PRESIDENT 2017-2021 U.S. Department of the Interior JANUARY 15, 2021 Report to the President Report to the President U.S. DEPARTMENT OF THE INTERIOR LETTER TO THE PRESIDENT The Department of the Interior (Department) has focused on improving the ways we serve the American public, while moving forward with your policy priorities. In doing so, the Department has made incredible progress furthering conservation stewardship, expanding opportunities to hunt and fish on public lands, improving core administrative functions, creating a common-sense regulatory regime, and enhancing our Nation’s energy independence. On behalf of the more than 65,000 Secretary David L. Bernhardt dedicated employees who work diligently across our Nation to accomplish important missions in service to the American people, I am pleased to present the Department’s Summary of Actions Report for 2017- 2021. This report highlights the Department’s major and historic achievements toward fulfilling your vision on behalf of all Americans. David L. Bernhardt Secretary of the Interior Page 1 Report to the President PURPOSE AND INTRODUCTION Secretary Bernhardt and First Lady Melania Trump at Grand Teton National Park From the beginning days of the Trump-Pence Administration, President Donald J. Trump gave clear direction to the Department of the Interior (Department, DOI, or Interior). He set priorities and ambitious goals, challenging Federal agencies through Governmentwide Executive orders, Presidential memoranda, and other actions to deliver better results for the American people. Interior has worked relentlessly to implement the President’s agenda for the betterment of our society and economy. -

W O 2016/201373 A1 15 December 2016 (15.12.2016) W I PO I P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date W O 2016/201373 A1 15 December 2016 (15.12.2016) W I PO I P C T (51) International Patent Classification: (74) Agents: QUEIROZ DE OLIVEIRA, Pierre et al.; Pepper A61K31/17(2006.01) A61P 25/00 (2006.01) Hamilton LLP, 500 Grant Street, Suite 5000, Pittsburgh, A61K 31/415 (2006.01) PA 15219-2507 (US). (21) International Application Number: (81) Designated States (unless otherwise indicated,for every PCT/US2016/037090 kind of national protection available): AE, AG, AL, AM, .) . AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (22) International Filng Date: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, 10 June 2016 (10.06.2016) DO, DZ, EC, EE, EG, ES, Fl, GB, GD, GE, GH, GM, GT, (25) Filing Language: English HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (26) Publication Language: English MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (30) Priority Data: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 62/174,983 12 June 2015 (12.06.2015) US SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 62/194,084 17 July 2015 (17.07.2015) US TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Monique Jannette Artist, Paralympian, and Former Civil Rights Lawyer
Texas Disability History Collection, University of Texas at Arlington Monique Jannette Artist, Paralympian, and former Civil Rights Lawyer Interview conducted by Mark Harris In 2016 in Dallas, Texas Disability Studies Minor Special Collections and Archives University of Texas at Arlington Copyright © 2016 by University of Texas at Arlington Libraries Biography Monique Jannette was born September 23, 1962 and graduated from Warren Travis White High School in Dallas, Texas, earned a Bachelor’s of Arts degree in Geology from the University of Texas at Arlington in 1987, and her Law Degree from Southern Methodist University in 1992. While in middle school and high school, she became interested in science and competitive diving. Jannette exceled at diving and traveled throughout the United States and other countries with diving teams. She earned a full scholarship to Southern Methodist University (SMU), but due to an accident shortly after graduation from high school in 1980, she became paraplegic. Starting in 1981, Jannette attended the University of Texas at Arlington (UTA), where she studied geology and participated in adaptive track and field under the direction of Jim Hayes. While attending UTA, she was selected to the 1988 U.S. Paralympic Track and Field team, and Table Tennis team in Seoul, South Korea. In 1992 she was selected again to compete with the U.S. Paralympic Team in Barcelona, Spain. Upon graduating from UTA, Jannette went back to SMU to study law. During her time there she collaborated with architects to make SMU more accessible to people with disabilities. After graduating law school, she and a partner began to practice civil rights law, helping the communities and business in and around the Dallas area. -

GPCR/G Protein
Inhibitors, Agonists, Screening Libraries www.MedChemExpress.com GPCR/G Protein G Protein Coupled Receptors (GPCRs) perceive many extracellular signals and transduce them to heterotrimeric G proteins, which further transduce these signals intracellular to appropriate downstream effectors and thereby play an important role in various signaling pathways. G proteins are specialized proteins with the ability to bind the nucleotides guanosine triphosphate (GTP) and guanosine diphosphate (GDP). In unstimulated cells, the state of G alpha is defined by its interaction with GDP, G beta-gamma, and a GPCR. Upon receptor stimulation by a ligand, G alpha dissociates from the receptor and G beta-gamma, and GTP is exchanged for the bound GDP, which leads to G alpha activation. G alpha then goes on to activate other molecules in the cell. These effects include activating the MAPK and PI3K pathways, as well as inhibition of the Na+/H+ exchanger in the plasma membrane, and the lowering of intracellular Ca2+ levels. Most human GPCRs can be grouped into five main families named; Glutamate, Rhodopsin, Adhesion, Frizzled/Taste2, and Secretin, forming the GRAFS classification system. A series of studies showed that aberrant GPCR Signaling including those for GPCR-PCa, PSGR2, CaSR, GPR30, and GPR39 are associated with tumorigenesis or metastasis, thus interfering with these receptors and their downstream targets might provide an opportunity for the development of new strategies for cancer diagnosis, prevention and treatment. At present, modulators of GPCRs form a key area for the pharmaceutical industry, representing approximately 27% of all FDA-approved drugs. References: [1] Moreira IS. Biochim Biophys Acta. 2014 Jan;1840(1):16-33. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -
Full Application
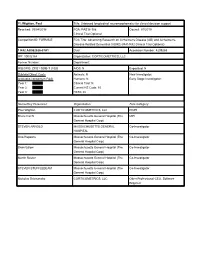
PI: Wighton, Paul Title: Unbiased longitudinal neuromorphometry for clinical decision support Received: 09/04/2018 FOA: PAS18-188 Council: 01/2019 Clinical Trial:Optional Competition ID: FORMS-E FOA Title: Advancing Research on Alzheimer's Disease (AD) and Alzheimer's- Disease-Related Dementias (ADRD) (R41/R42 Clinical Trial Optional) 1 R42 AG062026-01A1 Dual: blank Accession Number: 4209265 IPF: 10032164 Organization: CORTICOMETRICS, LLC Former Number: blank Department: blank IRG/SRG: ZRG1 SBIB-T (10)B AIDS: N Expedited: N Subtotal Direct Costs Animals: N New Investigator: blank (excludes consortium F&A) Humans: N Early Stage Investigator: blank Year 1: Clinical Trial: N Year 2: Current HS Code: 10 Year 3: HESC: N Senior/Key Personnel: Organization: Role Category: Paul Wighton CORTICOMETRICS, LLC PD/PI Bruce Fischl Massachusetts General Hospital (The MPI General Hospital Corp) STEVEN ARNOLD MASSACHUSETTS GENERAL Co-Investigator HOSPITAL Otto Rapalino Massachusetts General Hospital (The Co-Investigator General Hospital Corp) Brian Edlow Massachusetts General Hospital (The Co-Investigator General Hospital Corp) Martin Reuter Massachusetts General Hospital (The Co-Investigator General Hospital Corp) STEVEN STUFFLEBEAM Massachusetts General Hospital (The Co-Investigator General Hospital Corp) Nicholas Schmansky CORTICOMETRICS, LLC Other Professional-CEO, Software Engineer OMB Number: 4040-0001 Expiration Date: 10/31/2019 APPLICATION FOR FEDERAL ASSISTANCE 3. DATE RECEIVED BY STATE State Application Identifier SF 424 (R&R) blank blank 1. TYPE -

Synthesis of Novel Aporphine-Inspired Neuroreceptor Ligands
City University of New York (CUNY) CUNY Academic Works All Dissertations, Theses, and Capstone Projects Dissertations, Theses, and Capstone Projects 2-2016 Synthesis of Novel Aporphine-Inspired Neuroreceptor Ligands Nirav R. Kapadia Graduate Center, City University of New York How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/gc_etds/792 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] SYNTHESIS OF NOVEL APORPHINE-INSPIRED NEURORECEPTOR LIGANDS by Nirav R Kapadia A dissertation submitted to the Graduate Faculty in Chemistry in partial fulfillment of the requirements for the degree of Doctor of Philosophy, The City University of New York. 2016 © 2016 NIRAV R KAPADIA All Rights Reserved ii This manuscript has been read and accepted by the Graduate Faculty in Chemistry in satisfaction of the dissertation requirement for the degree of Doctor of Philosophy ____________________ _________________________ Date Dr. Wayne Harding Chair of Examining Committee ____________________ __________________________ Date Dr. Brian Gibney Executive Officer Dr. Adam Profit Dr. Shengping Zheng Dr. Wayne Harding Supervisory Committee THE CITY UNIVERSITY OF NEW YORK iii ABSTRACT Synthesis of Novel Aporphine-Inspired Neuroreceptor Ligands by Nirav R Kapadia Advisor: Dr. Wayne Harding Aporphines are a group of tetracyclic alkaloids that belong to the ubiquitous tetrahydroisoquinoline family. The aporphine template is known to be associated with a range of biological activities. Aporphines have been explored as antioxidants, anti-tuberculosis, antimicrobial and anticancer agents. Within the Central Nervous Systems (CNS), aporphine alkaloids are known to possess high affinity for several clinically valuable targets including dopamine receptors (predominantly D1 and D2), serotonin receptors (5-HT1A and 5-HT7) and α adrenergic receptors. -

To Consignors Hip Color & No
Index to Consignors Hip Color & No. Sex Name,Year Foaled Sire Dam Barn 7 Consigned by Ashview Farm LLC (Mr. and Mrs. Wayne G. Lyster III), Agent Broodmare 1780 b. m. Hurricane Victress, 1997 Sharp Victor Woody's Miss Broodmare prospect 1840 dk. b./br. m. Military Victress, 2002 Military Hurricane Victress Barn 9 Consigned by Blandford Stud (Padraig Campion), Agent Broodmare 1693 ch. m. Dance Trick, 1995 Diesis (GB) Performing Arts (IRE) Racing or broodmare prospect 1846 b. m. Missisipi Star (IRE), 2003 Mujahid Kicka Racing or stallion prospect 1874 b. c. Wafi City, 2004 E Dubai My Jody Racing prospect 1611 dk. b./br. f. unnamed, 2006 Roaring Fever Waywayanda Barn 2 Consigned by Bluewater Sales LLC, Agent I Yearling 1631 b. f. unnamed, 2007 Chapel Royal Alma Mater Barn 2 Consigned by Bluewater Sales LLC, Agent III Broodmare 1850 ch. m. Miss Phone Chatter, 2000 Phone Trick Passing My Way Broodmare prospect 1732 ch. m. Fancy Forever, 2003 Wild Rush Opinionated Lady Barn 2 Consigned by Bluewater Sales LLC, Agent IV Broodmare prospect 1678 dk. b./br. f. Classy Clarita, 2004 Mr. Greeley Link to Erin Barn 2 Consigned by Bluewater Sales LLC, Agent V Yearlings 1589 dk. b./br. c. unnamed, 2007 Offlee Wild Theatriken 1610 b. c. unnamed, 2007 Maria's Mon Way Beyond Barn 2 Consigned by Bluewater Sales LLC, Agent VI Racing or broodmare prospect 1694 gr/ro. f. Dancing Cherokee, 2004 Yonaguska Dancapade Barn 2 Consigned by Bluewater Sales LLC, Agent VII Yearling 1787 dk. b./br. f. unnamed, 2007 Offlee Wild Jettin Diplomacy Barn 2 Consigned by Bluewater Sales LLC, Agent VIII Broodmare 1754 b.