Criticality Experiments with Low Enriched U02 Fuel Rods in Water Containing Dissolved Gadolinium
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
(12) Patent Application Publication (10) Pub. No.: US 2008/0232532 A1 Larsen Et Al
US 20080232532A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0232532 A1 Larsen et al. (43) Pub. Date: Sep. 25, 2008 (54) APPARATUS AND METHOD FOR Related U.S. Application Data GENERATION OF ULTRA LOW MOMENTUM NEUTRONS (60) Provisional application No. 60/676,264, filed on Apr. 29, 2005. Provisional application No. 60/715,622, filed on Sep. 9, 2005. (76) Inventors: Lewis G. Larsen, Chicago, IL (US); Alan Widom, Brighton, MA (US) Publication Classification (51) Int. Cl. Correspondence Address: H05H 3/06 (2006.01) COOK, ALEX, MCFARRON, MANZO, (52) U.S. Cl. .............................................................. 376/108 CUMMINGS & MEHLER LTD (57) ABSTRACT SUTE 28SO Method and apparatus for generating ultra low momentum 2OO WESTADAMS STREET neutrons (ULMNS) using Surface plasmon polariton elec CHICAGO, IL 60606 (US) trons, hydrogen isotopes, Surfaces of metallic Substrates, col Appl. No.: 11/912,793 lective many-body effects, and weak interactions in a con (21) trolled manner. The ULMNs can be used to trigger nuclear PCT Filed: Apr. 28, 2006 transmutation reactions and produce heat. One aspect of the (22) present invention effectively provides a “transducer mecha (86) PCT NO.: PCT/US06/16379 nism that permits controllable two-way transfers of energy back-and-forth between chemical and nuclear realms in a S371(c)(1), Small-scale, low-energy, Scalable condensed matter system at (2), (4) Date: Oct. 26, 2007 comparatively modest temperatures and pressures. 1222222 Patent Application Publication Sep. 25, 2008 Sheet 1 of 8 US 2008/0232532 A1 Patent Application Publication Sep. 25, 2008 Sheet 3 of 8 US 2008/0232532 A1 & N. -

Discovery of Samarium, Europium, Gadolinium, and Terbium Isotopes
Discovery of Samarium, Europium, Gadolinium, and Terbium Isotopes E. May, M. Thoennessen∗ National Superconducting Cyclotron Laboratory and Department of Physics and Astronomy, Michigan State University, East Lansing, MI 48824, USA Abstract Currently, thirty-four samarium, thirty-four europium, thirty-one gadolinium, and thirty-one terbium isotopes have been observed and the discovery of these isotopes is discussed here. For each isotope a brief synopsis of the first refereed publication, including the production and identification method, is presented. arXiv:1201.4159v1 [nucl-ex] 19 Jan 2012 ∗Corresponding author. Email address: [email protected] (M. Thoennessen) Preprint submitted to Atomic Data and Nuclear Data Tables October 21, 2018 Contents 1. Introduction . 2 2. Discovery of 129−162Sm ................................................................................. 3 3. Discovery of 130−165Eu.................................................................................. 10 4. Discovery of 135−166Gd ................................................................................. 18 5. Discovery of 135−168Tb ................................................................................. 26 6. Summary ............................................................................................. 34 References . 34 Explanation of Tables . 41 7. Table 1. Discovery of samarium, europium, gadolinium, and terbium isotopes . 41 Table 1. Discovery of samarium, europium, gadolinium, and terbium isotopes. See page 41 for Explanation -

Neutron Capture Measurements and Resonance Parameters of Gadolinium
NUCLEAR SCIENCE AND ENGINEERING: 180, 86–116 (2015) Neutron Capture Measurements and Resonance Parameters of Gadolinium Y.-R. Kang and M. W. Lee Dongnam Institute of Radiological and Medical Sciences, Research Center Busan 619-953, Korea G. N. Kim* Kyungpook National University, Department of Physics Daegu 702-701, Korea T.-I. Ro Dong-A University, Department of Physics Busan 604-714, Korea Y. Danon and D. Williams Rensselaer Polytechnic Institute, Department of Mechanical, Aerospace, and Nuclear Engineering Troy, New York 12180-3590 and G. Leinweber, R. C. Block, D. P. Barry, and M. J. Rapp Bechtel Marine Propulsion Corporation, Knolls Atomic Power Laboratory P.O. Box 1072, Schenectady, New York 12301 Received June 6, 2014 Accepted July 15, 2014 http://dx.doi.org/10.13182/NSE14-80 Abstract – Neutron capture measurements were performed with the time-of-flight method at the Gaerttner LINAC Center at Rensselaer Polytechnic Institute (RPI) using isotopically enriched gadolinium (Gd) samples (155Gd, 156Gd, 157Gd, 158Gd, and 160Gd). The neutron capture measurements were made at the 25-m flight station with a 16-segment sodium iodide multiplicity detector. After the data were collected and reduced to capture yields, resonance parameters were obtained by a combined fitting of the neutron capture data for five enriched Gd isotopes and one natural Gd sample using the multilevel R-matrix Bayesian code SAMMY. A table of resonance parameters and their uncertainties is presented. We observed 2, 169, 96, and 1 new resonances in 154Gd, 155Gd, 157Gd, and 158Gd isotopes, respectively. Resonances in the ENDF/B-VII.0 evaluation that were not observed in the current experiment and could not be traced to a literature reference were removed. -

The Elements.Pdf
A Periodic Table of the Elements at Los Alamos National Laboratory Los Alamos National Laboratory's Chemistry Division Presents Periodic Table of the Elements A Resource for Elementary, Middle School, and High School Students Click an element for more information: Group** Period 1 18 IA VIIIA 1A 8A 1 2 13 14 15 16 17 2 1 H IIA IIIA IVA VA VIAVIIA He 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 2 Li Be B C N O F Ne 6.941 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 3 Na Mg IIIB IVB VB VIB VIIB ------- VIII IB IIB Al Si P S Cl Ar 22.99 24.31 3B 4B 5B 6B 7B ------- 1B 2B 26.98 28.09 30.97 32.07 35.45 39.95 ------- 8 ------- 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.47 58.69 63.55 65.39 69.72 72.59 74.92 78.96 79.90 83.80 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 5 Rb Sr Y Zr NbMo Tc Ru Rh PdAgCd In Sn Sb Te I Xe 85.47 87.62 88.91 91.22 92.91 95.94 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 6 Cs Ba La* Hf Ta W Re Os Ir Pt AuHg Tl Pb Bi Po At Rn 132.9 137.3 138.9 178.5 180.9 183.9 186.2 190.2 190.2 195.1 197.0 200.5 204.4 207.2 209.0 (210) (210) (222) 87 88 89 104 105 106 107 108 109 110 111 112 114 116 118 7 Fr Ra Ac~RfDb Sg Bh Hs Mt --- --- --- --- --- --- (223) (226) (227) (257) (260) (263) (262) (265) (266) () () () () () () http://pearl1.lanl.gov/periodic/ (1 of 3) [5/17/2001 4:06:20 PM] A Periodic Table of the Elements at Los Alamos National Laboratory 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Lanthanide Series* Ce Pr NdPmSm Eu Gd TbDyHo Er TmYbLu 140.1 140.9 144.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Actinide Series~ Th Pa U Np Pu AmCmBk Cf Es FmMdNo Lr 232.0 (231) (238) (237) (242) (243) (247) (247) (249) (254) (253) (256) (254) (257) ** Groups are noted by 3 notation conventions. -

Dynamics of Population of Gadolinium-156 Nuclei Energy Levels During Neutron Pumping of Isotope-Modified Gadolinium Oxide
Dynamics of population of gadolinium-156 nuclei energy levels during neutron pumping of isotope-modified gadolinium oxide Igor V. Shamanina, Mishik A. Kazaryanb, Sergei V. Bedenkoa, Vladimir.V. Knysheva, Vitaly I. Shamanina a National research Tomsk polytechnic university, prospekt Lenina, 30, 634050, Tomsk, Russia; b Physical institute RAS, Leninsky prospekt, 53, 119991, Moscow, Russia ABSTRACT The possibility of transformation of energy of fast and epithermal neutrons to energy of coherent photon radiation at the expense of a neutron pumping of the active medium formed by nucleus with long-living isomerous states is theoretically described. The channel of the nucleus formation in isomeric state as a daughter nucleus resulting from the nuclear reaction of neutron capture by a lighter nucleus is taken into consideration for the first time. The analysis of cross sections dependence of radiative neutron capture by the nuclei of gadolinium isotopes Gd155 and Gd156 is performed. As a result, it is stated that the speed of Gd156 nuclei formation exceeds the speed of their “burnup” in the neutron flux. It is provided by a unique combination of absorbing properties of two isotopes of gadolinium Gd155 and Gd156 in both thermal and resonance regions of neutron energy. The possibility of excess energy accumulation in the participating medium created by the nuclei of the pair of gadolinium isotopes Gd155 and Gd156 due to formation and storage of nuclei in isomeric state at radiative neutron capture by the nuclei of the stable isotope with a smaller mass is shown. It is concluded that when the active medium created by gadolinium nuclei is pumped by neutrons with the flux density of the order of 1013 cm-2·s-1, the condition of levels population inversion can be achieved in a few tens of seconds. -

Modern Physics
REVIEWS OF MODERN PHYSICS VoLUME 29, NuMBER 4 OcroBER, 1957 Synthesis of the Elements in Stars* E. MARGARET BURBIDGE, G. R. BURBIDGE, WILLIAM: A. FOWLER, AND F. HOYLE Kellogg Radiation1Laboratory, California Institute of Technology, and M aunt Wilson and Palomar Observatories, Carnegie Institution of Washington, California Institute of Technology, Pasadena, California "It is the stars, The stars above us, govern our conditions"; (King Lear, Act IV, Scene 3) but perhaps "The fault, dear Brutus, is not in our stars, But in ourselves," (Julius Caesar, Act I, Scene 2) TABLE OF CONTENTS Page I. Introduction ...............................................................· ............... 548 A. Element Abundances and Nuclear Structure. 548 B. Four Theories of the Origin of the Elements ............................................... 550 C. General Features of Stellar Synthesis ..................................................... 550 II. Physical Processes Involved in Stellar Synthesis, Their Place of Occurrence, and the Time-Scales Associated with Them ...............· ..................... · ................................. 551 A. Modes of Element Synthesis ............................................................. 551 B. Method of Assignment of Isotopes among Processes (i) to (viii) .............................. 553 C. Abundances and Synthesis Assignments Given in the Appendix. 555 D. Time-Scales for Different Modes of Synthesis .............................................. 556 III. Hydrogen Burning, Helium Burning, the a Process, -

Experimental Searches for Rare Alpha and Beta Decays
Experimental searches for rare alpha and beta decays P. Belli1;2;a, R. Bernabei1;2, F.A. Danevich3, A. Incicchitti4;5, and V.I. Tretyak3 1 Dip. Fisica, Universit`adi Roma \Tor Vergata", I-00133 Rome, Italy 2 INFN sezione Roma \Tor Vergata", I-00133 Rome, Italy 3 Institute for Nuclear Research, 03028 Kyiv, Ukraine 4 Dip. Fisica, Universit`adi Roma \La Sapienza", I-00185 Rome, Italy 5 INFN sezione Roma, I-00185 Rome, Italy Abstract. The current status of the experimental searches for rare alpha and beta decays is reviewed. Several interesting observations of alpha and beta decays, previously unseen due to their large half-lives (1015 − 1020 yr), have been achieved during the last years thanks to the improvements in the experimental techniques and to the underground locations of experiments that allows to suppress backgrounds. In particular, the list includes first observations of alpha decays of 151Eu, 180W (both to the ground state of the daughter nuclei), 190Pt (to excited state of the daughter nucleus), 209Bi (to the ground and excited 209 19 states of the daughter nucleus). The isotope Bi has the longest known half-life of T1=2 ≈ 10 yr relatively 115 115 to alpha decay. The beta decay of In to the first excited state of Sn (Eexc = 497:334 keV), recently observed for the first time, has the Qβ value of only (147 ± 10) eV, which is the lowest Qβ value known to-date. Searches and investigations of other rare alpha and beta decays (48Ca, 50V, 96Zr, 113Cd, 123Te, 178m2Hf, 180mTa and others) are also discussed. -
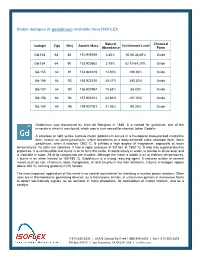
Stable Isotopes of Gadolinium Available from ISOFLEX
Stable isotopes of gadolinium available from ISOFLEX Natural Chemical Isotope Z(p) N(n) Atomic Mass Enrichment Level Abundance Form Gd-152 64 88 151.919789 0.20% 30.60-34.80% Oxide Gd-154 64 90 153.920862 2.18% 52.10-64.20% Oxide Gd-155 64 91 154.922619 14.80% >90.00% Oxide Gd-156 64 92 155.922120 20.47% ≥93.30% Oxide Gd-157 64 93 156.923957 15.65% 88.40% Oxide Gd-158 64 94 157.924101 24.84% >97.30% Oxide Gd-160 64 96 159.927051 21.86% 98.20% Oxide Gadolinium was discovered by Jean de Marignac in 1880. It is named for gadolinite, one of the minerals in which it was found, which was in turn named for chemist Johan Gadolin. A colorless or light yellow lustrous metal, gadolinium occurs in a hexagonal close-packed crystalline form, known as alpha-gadolinium, which transforms to a body-centered cubic allotropic form, beta- gadolinium, when it reaches 1262 ºC. It exhibits a high degree of magnetism, especially at lower temperatures. Its salts are colorless. It has a vapor pressure of 9.0 torr at 1800 ºC. It also has superconductive properties. It is combustible and burns in air to form the oxide. It reacts slowly in water, is soluble in dilute acid, and is insoluble in water. All of its compounds are trivalent. Although the metal is stable in air at ordinary temperatures, it burns in air when heated to 150-180 ºC. Gadolinium is a strong reducing agent. It reduces oxides of several metals such as iron, chromium, lead, manganese, tin and zirconium into their elements. -

A Periodic Table of the Elements at Los Alamos National Laboratory Los Alamos National Laboratory's Chemistry Division Presents Periodic Table of the Elements
A Periodic Table of the Elements at Los Alamos National Laboratory Los Alamos National Laboratory's Chemistry Division Presents Periodic Table of the Elements A Resource for Elementary, Middle School, and High School Students Click an element for more information: Group** Period 1 18 IA VIIIA 1A 8A 1 2 13 14 15 16 17 2 1 H IIA IIIA IVA VA VIAVIIA He 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 2 Li Be B C N O F Ne 6.941 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 3 Na Mg IIIB IVB VB VIB VIIB ------- VIII IB IIB Al Si P S Cl Ar 22.99 24.31 3B 4B 5B 6B 7B ------- 1B 2B 26.98 28.09 30.97 32.07 35.45 39.95 ------- 8 ------- 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.47 58.69 63.55 65.39 69.72 72.59 74.92 78.96 79.90 83.80 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 5 Rb Sr Y Zr NbMo Tc Ru Rh PdAgCd In Sn Sb Te I Xe 85.47 87.62 88.91 91.22 92.91 95.94 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 6 Cs Ba La* Hf Ta W Re Os Ir Pt AuHg Tl Pb Bi Po At Rn 132.9 137.3 138.9 178.5 180.9 183.9 186.2 190.2 190.2 195.1 197.0 200.5 204.4 207.2 209.0 (210) (210) (222) 87 88 89 104 105 106 107 108 109 110 111 112 114 116 118 7 Fr Ra Ac~RfDb Sg Bh Hs Mt --- --- --- --- --- --- (223) (226) (227) (257) (260) (263) (262) (265) (266) () () () () () () http://pearl1.lanl.gov/periodic/ (1 of 2) [5/10/2001 3:08:31 PM] A Periodic Table of the Elements at Los Alamos National Laboratory 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Lanthanide Series* Ce Pr NdPmSm Eu Gd TbDyHo Er TmYbLu 140.1 140.9 144.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Actinide Series~ Th Pa U Np Pu AmCmBk Cf Es FmMdNo Lr 232.0 (231) (238) (237) (242) (243) (247) (247) (249) (254) (253) (256) (254) (257) ** Groups are noted by 3 notation conventions. -

Foia/Pa-2015-0050
Acknowledgements The following were the members of ICRP Committee 2 who prepared this report. J. Vennart (Chairman); W. J. Bair; L. E. Feinendegen; Mary R. Ford; A. Kaul; C. W. Mays; J. C. Nenot; B. No∈ P. V. Ramzaev; C. R. Richmond; R. C. Thompson and N. Veall. The committee wishes to record its appreciation of the substantial amount of work undertaken by N. Adams and M. C. Thorne in the collection of data and preparation of this report, and, also, to thank P. E. Morrow, a former member of the committee, for his review of the data on inhaled radionuclides, and Joan Rowley for secretarial assistance, and invaluable help with the management of the data. The dosimetric calculations were undertaken by a task group, centred at the Oak Ridge Nntinnal-..._._.__. ---...“..,-J,1 .nhnmtnrv __ac ..,.I_.,“.fnllnwr~ Mary R. Ford (Chairwoman), S. R. Bernard, L. T. Dillman, K. F. Eckerman and Sarah B. Watson. Committee 2 wishes to record its indebtedness to the task group for the completion of this exacting task. The data given in this report are to be used together with the text and dosimetric models described in Part 1 of ICRP Publication 30;’ the chapters referred to in this preface relate to that report. In order to derive values of the Annual Limit on Intake (ALI) for radioisotopes of scandium, the following assumptions have been made. In the metabolic data for scandium, a fraction 0.4 of the element in the transfer compartment is translocated to the skeleton. It is assumed thit this scandium in the skeleton is distributed between cortical bone, trabecular bone and red marrow in proportion to their respective masses. -

A Periodic Table of the Elements at Los Alamos National Laboratory Los Alamos National Laboratory's Chemistry Division Presents Periodic Table of the Elements
A Periodic Table of the Elements at Los Alamos National Laboratory Los Alamos National Laboratory's Chemistry Division Presents Periodic Table of the Elements A Resource for Elementary, Middle School, and High School Students Click an element for more information: Group** Period 1 18 IA VIIIA 1A 8A 1 2 13 14 15 16 17 2 1 H IIA IIIA IVA VA VIA VIIA He 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 2 Li Be B C N O F Ne 6.941 9.012 10.81 12.01 14.0116.00 19.00 20.18 11 12 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 3 Na Mg IIIB IVB VB VIB VIIB------- VIII ------ IB IIB Al Si P S Cl Ar 22.99 24.31 3B 4B 5B 6B 7B - 1B 2B 26.98 28.09 30.9732.07 35.45 39.95 ------- 8 ------- 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.10 40.08 44.96 47.8850.94 52.00 54.94 55.85 58.47 58.6963.5565.39 69.72 72.59 74.9278.96 79.90 83.80 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 5 Rb Sr Y Zr NbMo Tc Ru Rh Pd AgCd In Sn Sb Te I Xe 85.47 87.62 88.91 91.2292.91 95.94 (98) 101.1 102.9 106.4107.9112.4 114.8 118.7 121.8127.6 126.9 131.3 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 6 Cs Ba La* Hf Ta W Re Os Ir Pt AuHg Tl Pb Bi Po At Rn 132.9 137.3 138.9 178.5180.9 183.9 186.2 190.2 190.2 195.1197.0200.5 204.4 207.2 209.0 (210) (210) (222) 87 88 89 104 105 106 107 108 109 110 111 112 114 116 118 7 Fr Ra Ac~ Rf Db Sg Bh Hs Mt --- --- --- --- --- --- (223) (226) (227) (257) (260) (263) (262) (265) (266) () () () () () () http://periodic.lanl.gov/default.htm (1 of 3) [10/24/2001 5:40:02 PM] A Periodic Table of the Elements at Los Alamos National Laboratory 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Lanthanide Series* Ce Pr NdPmSm Eu Gd Tb DyHo Er Tm Yb Lu 140.1 140.9144.2 (147) 150.4 152.0 157.3 158.9162.5164.9 167.3 168.9 173.0175.0 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Actinide Series~ Th Pa U Np Pu AmCmBk Cf Es FmMdNo Lr 232.0 (231) (238) (237) (242) (243) (247) (247) (249) (254) (253) (256) (254) (257) ** Groups are noted by 3 notation conventions. -

349 57201233213.Pdf
Spectrochimica Acta Part B 64 (2009) 1259–1265 Contents lists available at ScienceDirect Spectrochimica Acta Part B journal homepage: www.elsevier.com/locate/sab Isotope ratio analysis on micron-sized particles in complex matrices by Laser Ablation-Absorption Ratio Spectrometry B.A. Bushaw a, N.C. Anheier Jr. b,⁎ a Fundamental and Computational Sciences Directorate, Pacific Northwest National Laboratory, Richland, WA 99352, United States b National Security Directorate, Pacific Northwest National Laboratory, Richland, WA 99352, United States article info abstract Article history: Laser ablation has been combined with dual tunable diode laser absorption spectrometry to measure Received 25 August 2009 152Gd:160Gd isotope ratios in micron-size particles. The diode lasers are tuned to specific isotopes in two Accepted 13 October 2009 different atomic transitions at 405.9 nm (152Gd) and 413.4 nm (160Gd) and directed collinearly through the Available online 18 October 2009 laser ablation plume, separated on a diffraction grating, and detected with photodiodes to monitor transient absorption signals on a shot-by-shot basis. The method has been characterized first using Gd metal foil and Keywords: then with particles of GdCl ·xH 0 as binary and ternary mixtures with 152Gd:160Gd isotope ratios ranging Laser ablation 3 2 from 0.01 to 0.43. These particulate mixtures have been diluted with Columbia River sediment powder (SRM Diode laser absorption Isotope ratio 4350B) to simulate environmental samples and we show the method is capable of detecting a few highly- Particulate enriched particles in the presence of a N100-fold excess of low-enrichment particles, even when the Gd- Gadolinium bearing particles are a minor component (0.08%) in the SRM powder and widely dispersed (1178 particles detected in 800,000 ablation laser shots).