Cellular Plasticity: a Mechanism for Homeostasis in the Kidney
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Kidney, Ureter, Urinary Bladder & Urethra
Kidney, Ureter, Urinary bladder & Urethra Red: important. Black: in male|female slides. Gray: notes|extra. Editing file ➢ OBJECTIVES • The microscopic structure of the renal cortex and medulla. • The histology of renal corpuscle, proximal and distal tubules, loop of Henle, and collecting tubules & ducts. • The histological structure of juxtaglomerular apparatus. • The functional structures of the different parts of the kidney. • The microscopic structure of the Renal pelvis and ureter. • The microscopic structure of the urinary bladder and male and female urethra Histology team 437 | Renal block | All lectures ➢ KIDNEY o Cortex: Dark brown and granular. Content of cortex (renal corpuscle, PCT, loop of Henle, DCT, part of collecting tubule) o Medulla: 6-12 pyramid-shape regions (renal pyramids) content of medulla ( collecting duct, loop of Henle, collecting tubule) o The base of pyramid is toward the cortex (cortico-medullary border) o The apex (renal papilla) toward the hilum, it is perforated by 12 openings of the ducts of Bellini (Papillary “collecting” ducts) in region called area cribrosa. o The apex is surrounded by a minor calyx. o 3 or 4 minor calyces join to form 3 or 4 major calyces that form renal pelvis. o Pyramids are separated by cortical columns of Bertin (renal column) ➢ URINIFEROUS TUBULE o It is the functional unit of the kidney. o Is formed of: 1- Nephron. 2-Collecting tubule. o The tubules are densely packed. o The tubules are separated by thin stroma and basal lamina. Histology team 437 | Renal block | All lectures ➢ NEPHRON o There are 2 types of nephrons: a- Cortical nephrons. b- Juxtamedullary nephrons. -
Urinary System
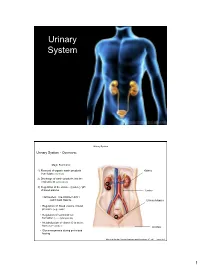
Urinary System Urinary System Urinary System - Overview: Major Functions: 1) Removal of organic waste products Kidney from fluids (excretion) 2) Discharge of waste products into the environment (elimination) 1 3) Regulation of the volume / [solute] / pH 3 of blood plasma Ureter HOWEVER, THE KIDNEY AIN’T JUST FOR PEE’IN… Urinary bladder • Regulation of blood volume / blood pressure (e.g., renin) • Regulation of red blood cell formation (i.e., erythropoietin) 2 • Metabolization of vitamin D to active form (Ca++ uptake) Urethra • Gluconeogenesis during prolonged fasting Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.1 1 Urinary System Renal ptosis: Kidneys drop to lower position due Functional Anatomy - Kidney: to loss of perirenal fat Located in the superior lumbar “Bar of soap” region 12 cm x 6 cm x 3 cm 150 g / kidney Layers of Supportive Tissue: Renal fascia: Peritoneal cavity Outer layer of dense fibrous connective tissue; anchors kidney in place Perirenal fat capsule: Fatty mass surrounding kidney; cushions kidney against blows Fibrous capsule: Transparent capsule on kidney; prevents infection of kidney from local tissues Kidneys are located retroperitoneal Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.2 Urinary System Functional Anatomy - Kidney: Pyelonephritis: Inflammation of the kidney Pyramids appear striped due to parallel arrangement of capillaries / collecting tubes Renal cortex Renal medulla Renal pyramids Renal papilla Renal columns Renal hilum Renal pelvis • Entrance for blood vessels -

Il Sistema Genito-Urinario
Ingegneria delle tecnologie per la salute Fondamenti di anatomia e istologia Il sistema genito-urinario Ingegneria delle tecnologie per la salute Fondamenti di anatomia e istologia Il sistema urinario Functions The role of urinary system: • storing urine until a convenient time for disposal • providing the anatomical structures to transport this waste liquid to the outside of the body • cleansing the blood and ridding the body of wastes • regulation of pH • regulation of blood pressure • regulation of the concentration of solutes in the blood • regulation of the concentration of red blood cells by producing erythropoietin (EPO) in the kidney • perform the final synthesis step of vitamin D production in the kidney If the kidneys fail, these functions are compromised or lost altogether, with devastating effects on homeostasis. The affected individual might experience weakness, lethargy, shortness of breath, anemia, widespread edema (swelling), metabolic acidosis, rising potassium levels, heart arrhythmias, and more. Each of these functions is vital to your well- being and survival. Urine The urinary system’s ability to filter the blood resides in about 2 to 3 million tufts of specialized capillaries—the glomeruli—distributed more or less equally between the two kidneys. Because the glomeruli filter the blood based mostly on particle size, large elements like blood cells, platelets, antibodies, and albumen are excluded. The glomerulus is the first part of the nephron, which then continues as a highly specialized tubular structure responsible for creating the final urine composition. The glomeruli create about 200 liters of filtrate every day, yet you excrete less than two liters of waste you call urine. -

The Morphology of the Juxtaglomerular Apparatus (JGA
Okajimas Folia Anat. Jpn., 63(6): 393-406, March 1987 Fine Structural Changes in the Three-Dimensional Structure of the Rat Juxtaglomerular Apparatus in Response to Water Deprivation By Sumie KIDOKORO Department of Anatomy, Yokohama City University School of Medicine, Kanazawaku, Yokohama, 236 Japan -Received for Publication, December 26, 1986- Key Words: juxtaglomerular apparatus, reconstruction, ultrastructure, water deprivation, rat Summary: Morphological changes in the juxtaglomerular apparatus (JGA) after water de- privation, especially those in the spatial relationships among the structural components of the JGA were investigated by electron microscopy of serial sections and the three-dimen- sional reconstruction. The most remarkable changes were observed after 1-day-water depriva- tion, i.e. the secretory granule-containing cell layer in the afferent arterioles was markedly increased in extent, and the ratio of contact area between the Goormaghtigh cells (GoCs) and the macula densa of the distal tubule to the whole surface of the GoC field was signifi- cantly reduced. A possible role of the GoCs in function of the JGA was discussed. The morphology of the juxtaglomerular a functional system for tubulo-glomerular apparatus (JGA) has been intensively studied feedback mechanism. It has been described by various approaches, including three- that the JGCs are also found in the efferent dimensional study using reconstruction arteriole as well as in the extraglomerular of serial sections (Barajas & Latta, '63; mesangial cells in some occasions. -

Cancer and the Kidney
Cancer and the Kidney DONALD E. OKEN, M.D . Professor of Medicine, and Chairman, Division of Nephrology, Medical College of Virginia, Health Sciences Division of Virginia Commonwealth University, Richmond, Virginia Cancer of the kidney is associated with a bewil tablished and offers at least a gleam of hope for those dering array of extrarenal symptoms, and conversely, in whom pulmonary metastases are found. tumors far removed from the kidney produce in Perhaps equally remarkable is the finding of dis triguing renal functional abnormalities. tant metastases many years after a renal cell carci A variety of extrarenal complications are seen noma is removed surgically. The longest recorded with hypernephromas, most of which rarely accom survival between a diagnosis of renal carcinoma and pany Wilms tumors which grow rapidly and generally the eventual death of a patient whose neoplasm was occur before the age of 7. Wilms tumors are quite considered inoperable and left in place is 37 years. 1 susceptible to radiation therapy and surgery, and are Many patients have been reported to develop metas to be strongly suspected when hypertension and an tases 5, JO, and even 25 years after the resection of a abdominal mass are found in a small child. Unless hypernephroma. I have seen a patient who developed treated, they rapidly cause death and usually leave "solitary" metastases sequentially over a 19-year pe little opportunity for the patient to develop the strik riod before he succumbed. Unfortunately, while such ing extrarenal manifestations seen with hyper cases stand out, metastases appear earlier in most nephroma. patients and lead to death within two years in one Among the fascinating complications of hyper third of patients. -

Glomus Tumor of Renal Pelvis: a Case Report and Review of the Literature
Human Pathology (2005) 36, 299–302 www.elsevier.com/locate/humpath Case studies Glomus tumor of renal pelvis: a case report and review of the literature Mehsati Herawi MD, PhDa, Anil V. Parwani MD, PhDa, Donald Edlow MDd, James K. Smolev MDe, Jonathan I. Epstein MDa,b,c,* aDepartment of Pathology, The Johns Hopkins University School of Medicine, Baltimore, MD 21205-2100, USA bDepartment of Urology, The Johns Hopkins University School of Medicine, Baltimore, MD 21205-2100, USA cDepartment of Oncology, The Johns Hopkins University School of Medicine, Baltimore, MD 21205-2100, USA dDepartment of Pathology, Good Samaritan Hospital, Baltimore, MD 21239, USA eDepartment of Urology, Good Samaritan Hospital, Baltimore, MD 21239, USA Keywords: Summary Glomus tumors are uncommon benign perivascular neoplasms that have rarely been Kidney; described outside of their usual peripheral soft tissue sites. We report a unique case of glomus tumor of Renal pelvis; the renal pelvis in a 53-year-old woman who presented with microscopic hematuria associated with Ureter; obstruction of the ureteropelvic junction and marked hydronephrosis. At initial gross examination, the Glomus tumor tumor mimicked a urothelial carcinoma. D 2005 Elsevier Inc. All rights reserved. Glomus tumors represent less than 2% of soft tissue 1. Case report tumors [1,2]. They most often occur in the extremities, typically in the subungual region of the fingers. They only A 53-year-old woman presented with a vague discomfort rarely involve the internal organs such as mediastinum, lung, in her right flank and with microscopic hematuria. Imaging trachea, and stomach [2,3]. Reports of glomus tumors in the studies including an abdominal computed tomography scan genitourinary regions are extremely rare and involve mostly and a retrograde pyelogram demonstrated a solid mass clitoris, vagina, cervix, and the periurethral soft tissue [4-6]. -

Kidney Solid Tumor Rules
Kidney Equivalent Terms and Definitions C649 (Excludes lymphoma and leukemia M9590 – M9992 and Kaposi sarcoma M9140) Introduction Note 1: Tables and rules refer to ICD-O rather than ICD-O-3. The version is not specified to allow for updates. Use the currently approved version of ICD-O. Note 2: 2007 MPH Rules and 2018 Solid Tumor Rules are used based on date of diagnosis. • Tumors diagnosed 01/01/2007 through 12/31/2017: Use 2007 MPH Rules • Tumors diagnosed 01/01/2018 and later: Use 2018 Solid Tumor Rules • The original tumor diagnosed before 1/1/2018 and a subsequent tumor diagnosed 1/1/2018 or later in the same primary site: Use the 2018 Solid Tumor Rules. Note 3: Renal cell carcinoma (RCC) 8312 is a group term for glandular (adeno) carcinoma of the kidney. Approximately 85% of all malignancies of the kidney C649 are RCC or subtypes/variants of RCC. • See Table 1 for renal cell carcinoma subtypes/variants. • Clear cell renal cell carcinoma (ccRCC) 8310 is the most common subtype/variant of RCC. Note 4: Transitional cell carcinoma rarely arises in the kidney C649. Transitional cell carcinoma of the upper urinary system usually arises in the renal pelvis C659. Only code a transitional cell carcinoma for kidney in the rare instance when pathology confirms the tumor originated in the kidney. Note 5: For those sites/histologies which have recognized biomarkers, the biomarkers are most frequently used to target treatment. Biomarkers may identify the histologic type. Currently, there are clinical trials being conducted to determine whether these biomarkers can be used to identify multiple primaries. -

Macula Densa Sensing and Signaling Mechanisms of Renin Release
SCIENCE IN RENAL MEDICINE www.jasn.org Macula Densa Sensing and Signaling Mechanisms of Renin Release Ja´nos Peti-Peterdi* and Raymond C. Harris† *Departments of Physiology and Biophysics and Medicine, Zilkha Neurogenetic Institute, University of Southern California, Los Angeles, California; and †Division of Nephrology, Department of Medicine, Vanderbilt University School of Medicine, Nashville, Tennessee ABSTRACT 8 Macula densa cells in the distal nephron, according to the classic paradigm, are salt release of PGE2. PGE2 acts on EP2 and sensors that generate paracrine chemical signals in the juxtaglomerular apparatus EP4 receptors in juxtaglomerular cells to control vital kidney functions, including renal blood flow, glomerular filtration, and causes renin release (Figure 1B).10 In and renin release. Renin is the rate-limiting step in the activation of the renin- addition to COX-2-derived prostaglan- angiotensin system, a key modulator of body fluid homeostasis. Here, we discuss dins, the neural isoform of nitric oxide recent advances in understanding macula densa sensing and suggest these cells, in synthases, which is selectively expressed addition to salt, also sense various chemical and metabolic signals in the tubular in macula densa cells,11 is critical in the environment that directly trigger renin release. tubuloglomerular feedback and renin signaling cascade.2,12,13 The paracrine J Am Soc Nephrol 21: 1093–1096, 2010. doi: 10.1681/ASN.2009070759 chemical signals of macula densa-medi- ated inhibition of renin release include ATP and adenosine.1–3,14 The juxtaglomerular apparatus in the renal juxtaglomerular apparatus-glomerular Besides the well-known NKCC2 co- cortex represents a major structural com- complex. -
Histology of Urinary System
HISTOLOGY OF URINARY SYSTEM Histology Departement FK USU August 20020099 Urinary System : Kidney, urinary passages Passages include: Calyces Renal Pelvis Ureter Accessory parts include: Urinary bladder Urethra Kidney : Flattened, bean-shaped ~4.5 inches long Capsule : thin, fibrous, weakly attached (mostly collagenous fibers) Interstitial C.T. – scant; entirely reticular tissue Hilus: slit-like orifice – opens into expanded renal sinus , a flattened cavity Renal sinus filled with: renal pelvis (expanded ureter); fat; C.T.; blood vessels; nerves Kidney crosscross--sectionsection Renal Pelvis: Subdivided into 2 or 3 major calyces Major calyces)(1 )(1subdivide into 7 – 10 minor calyces)(2 )(2 Minor calyces fit over a renal papilla)(3 )(3 11 22 33 Kidney interior: largely parenchyma Parenchyma: many long, tortuous secretory canals ( nephrons ) Excretory ducts of nephrons discharge into minor calyces Nephron + excretory duct = uriniferous tubule Parenchyma divisible into Cortex & Medulla Medulla (gray when fresh) composed of 10 – 15 renal pyramids 2 to 3 pyramids fuse: end in one papilla i.e., 6 to 14 papillae / kidney (human) Pyramids appear striate (tubules are straight) Cortex ( brownish in life) Inner border irregular Over base of pyramid; between pyramids ( Renal columns of Bertin ) Nephron (Uriniferous Tubule) – Kidney is a compound gland Uriniferous tubule composed of two parts: Nephron & Collecting tubule Nephron: The physiological unit of the kidney used for filtration of blood and reabsorption and secretion of materials Unbranched; -

RENAL SYSTEM- NOTES II Learning Objectives by the End of This Section
RENAL SYSTEM- NOTES II Learning Objectives By the end of this section, you will be able to: • Distinguish the histological differences between the renal cortex and medulla • Describe the structure of the filtration membrane • Identify the major structures and subdivisions of the renal corpuscles, renal tubules, and renal capillaries • Discuss the function of the peritubular capillaries and vasa recta • Identify the location of the juxtaglomerular apparatus and describe the cells that line it • Describe the histology of the proximal convoluted tubule, loop of Henle, distal convoluted tubule, and collecting ducts The renal structures that conduct the essential work of the kidney cannot be seen by the naked eye. Only a light or electron microscope can reveal these structures. Even then, serial sections and computer reconstruction are necessary to give us a comprehensive view of the functional anatomy of the nephron and its associated blood vessels. NEPHRONS: THE FUNCTIONAL UNIT Nephrons take a simple filtrate of the blood and modify it into urine. Many changes take place in the different parts of the nephron before urine is created for disposal. The term forming urine will be used hereafter to describe the filtrate as it is modified into true urine. The principle task of the nephron population is to balance the plasma to homeostatic set points and excrete potential toxins in the urine. They do this by accomplishing three principle functions—filtration, reabsorption, and secretion. They also have additional secondary functions that exert control in three areas: blood pressure (via production of renin ), red blood cell production (via the hormone EPO), and calcium absorption (via conversion of calcidiol into calcitriol, the active form of vitamin D). -

Juxtaglomerular Cell Tumor: Reviewing a Cryptic Cause of Surgically Correctable Hypertension
Review Curr Urol 2019;13:7–12 Received: July 9, 2018 DOI: 10.1159/000499301 Accepted: July 22, 2018 Published online: September 10, 2019 Juxtaglomerular Cell Tumor: Reviewing a Cryptic Cause of Surgically Correctable Hypertension Rafid Inama Jason Gandhia,b Gunjan Joshic Noel L. Smithd Sardar Ali Khana,e aDepartment of Physiology and Biophysics, Stony Brook Renaissance University School of Medicine, Stony Brook, NY, USA; bMedical Student Research Institute, St. George’s University School of Medicine, Grenada, West Indies; cDepartment of Internal Medicine, Stony Brook Southampton Hospital, Southampton, NY; dFoley Plaza Medical, New York, NY; eDepartment of Urology, Stony Brook Renaissance University School of Medicine, Stony Brook, NY, USA Key Words Introduction Juxtaglomerular cell tumor • Reninoma • Renin • Secondary hypertension • Partial nephrectomy Juxtaglomerular cell tumor (JGCT), or reninoma, is a rare disease resulting from renin-induced hypertension. Dysfunction in juxtaglomerular cells lead to the over- Abstract expression of renin. Although presented with limited Juxtaglomerular cell tumor (JGCT), or reninoma, is a typi- cases, diagnosis of JGCT occurs within young adults and cally benign neoplasm generally affecting adolescents and adolescents with some occurrences within young chil- young adults due to modified smooth muscle cells from the dren [1, 2]. JGCT-induced hypertension is the primary afferent arteriole of the juxtaglomerular apparatus. Patients symptom with other rare occurrences such as myocar- experience symptoms related to hypertension and hypoka- dial infarction. JGCT can coexist with various forms of lemia due to renin-secretion by the tumor. MRI, PET, CT, and cancer, indirectly enhancing those various types of can- renal vein catheterizations can be used to capture JGCTs, cers. -

Distal Convoluted Tubule (DCT)
Distal convoluted tubule (DCT) Simple Short cuboid epithelium Large lumen Short microvillus No brush border Base: basal ridge + mitochondria Macula densa Distal convoluted tubule (DCT) functions: Reabsorbtion of Na+ / water (by aldestron ) Reabsorbtion of bicarbonate Reabsorbtion of Ca+ Secretion of hydrogen / ammonium ( acid-base balance) Secretion K+ PCT & DCT Collecting tubule/duct Chief cell: Cuboid / light cytoplasm / basal ridge / apical single cilia / Intercalated or dark cell: Dark cuboid / apical microvillus / mitochondria Collecting tube – collecting duct – duct of bellini (area cribrosa) – minor calyxes Single cilia in chief cell: Have polycystin protein Functions: Ca+ channel Adhesion protein in cell to cell junction Attach cell to basal membrane Chief cell : Collecting tubule/duct Reabsorption Na+ / water Secretion K+ Dark cell : Reabsorption K Secretion H+ / HCO3 Juxtaglomerular apparatus Located in vascular pole Consist of 3 parts: Macula densa Juxtaglomerular cell External mesangial cell Macula densa: Juxtaglomerular apparatus Modified cell of DCT Located around afferent arteriole Long / dark cell Central or apical nucleus Apical microvillus susceptible to low Na+ concentration Functions: Renin secretion / angiotensin I to angiotensin II convertor enzyme Juxtaglomerular cells: Modified smooth muscle cells of afferent arteriole Round nucleus Secretory granules in cytoplasm Rough ER / Golgi Juxtaglomerular apparatus External mesangial cell: polkissen / lacis cells Occupied space between afferent & efferent arterioles & macula